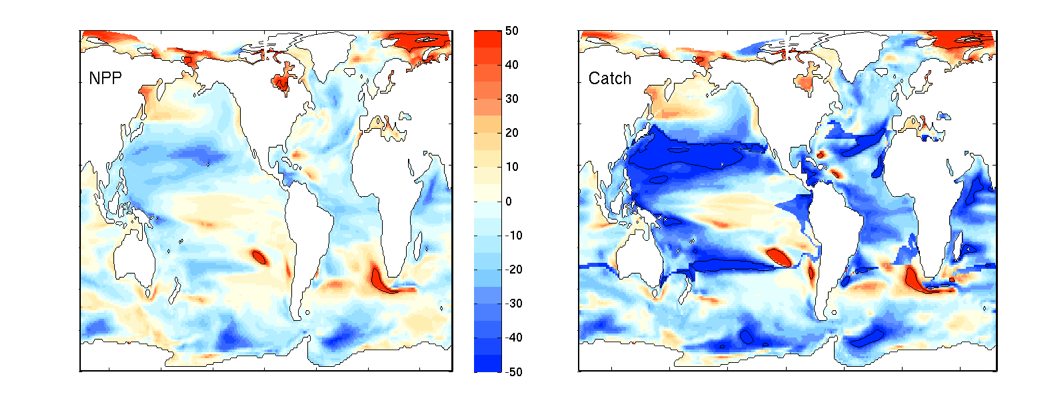
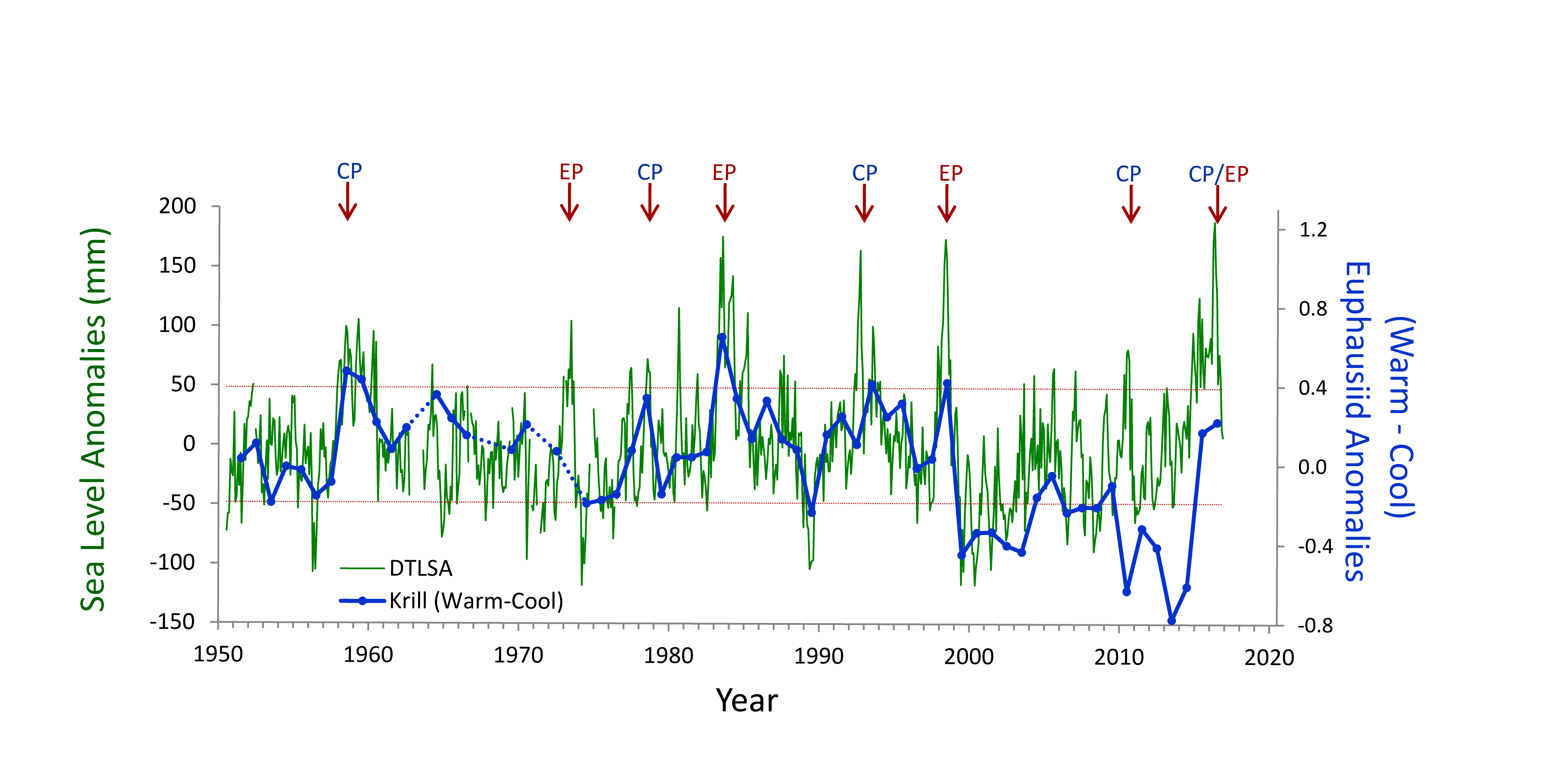
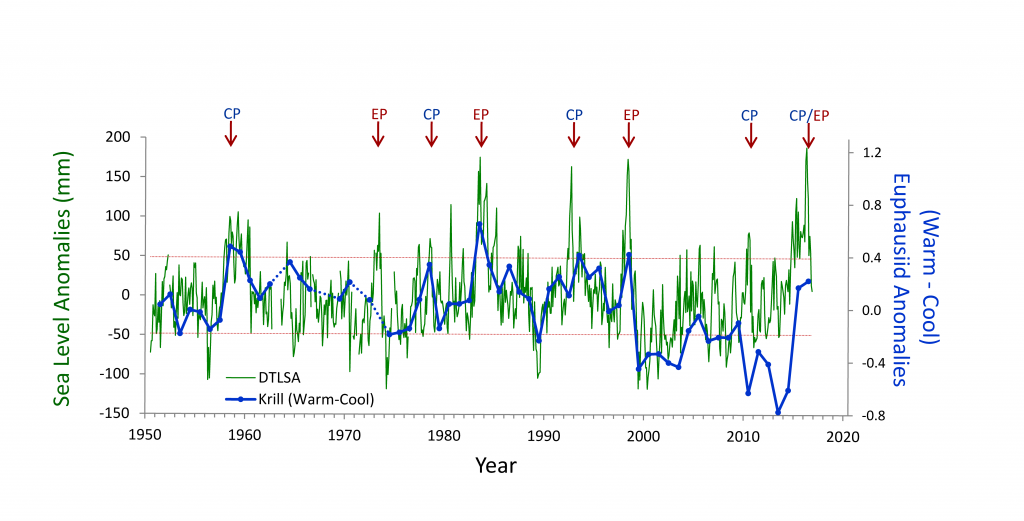
Turbulence is known to be a primary determinant of plankton fitness and succession. However, open questions remain about whether phytoplankton can actively respond to turbulence and, if so, how rapidly they can adapt to it. Recent experiments have revealed that phytoplankton can behaviorally respond to turbulent cues with a rapid change in shape, and this response occurs over a few minutes. This challenges a fundamental paradigm in oceanography that phytoplankton are passively at the mercy of turbulence.
Phytoplankton are photosynthetic microorganisms that form the base of most aquatic food webs, impact global biogeochemical cycles, and produce half of the world’s oxygen. Many species of phytoplankton are motile and migrate in response to gravity and light levels: Upward toward light during the day to photosynthesize and downward at night toward higher nutrient concentrations. Disruption of this diurnal migratory strategy is an important contributor to the succession between motile and non-motile species when conditions become more turbulent. However, this classical view neglects the possibility that motile species can actively respond in an effort to avoid layers of strong turbulence. A recent study by Sengupta, Carrara and Stocker, published in Nature has shown that some raphidophyte and dinoflagellate phytoplankton can actively diversify their migratory strategy in response to hydrodynamic cues characteristic of overturning by the smallest turbulent eddies in the ocean. Laboratory experiments in which cells experienced repeated overturning with timescales and statistics representative of ocean turbulence revealed that over timescales as short as ten minutes, an upward-swimming population split into two subpopulations, one swimming upward and one swimming downward. Quantitative morphological analysis of the harmful algal bloom-forming raphidophyte Heterosigma akashiwo revealed that this behavior was accompanied by a change in cell shape, wherein the cells that changed their swimming direction did so by going from an asymmetric pear shape to a more symmetric egg shape. A model of cell mechanics showed that the magnitude of this shift was minute, yet sufficient to invert the cells’ preferential swimming direction. The results highlight the advanced level of control that phytoplankton have on their migratory behavior.
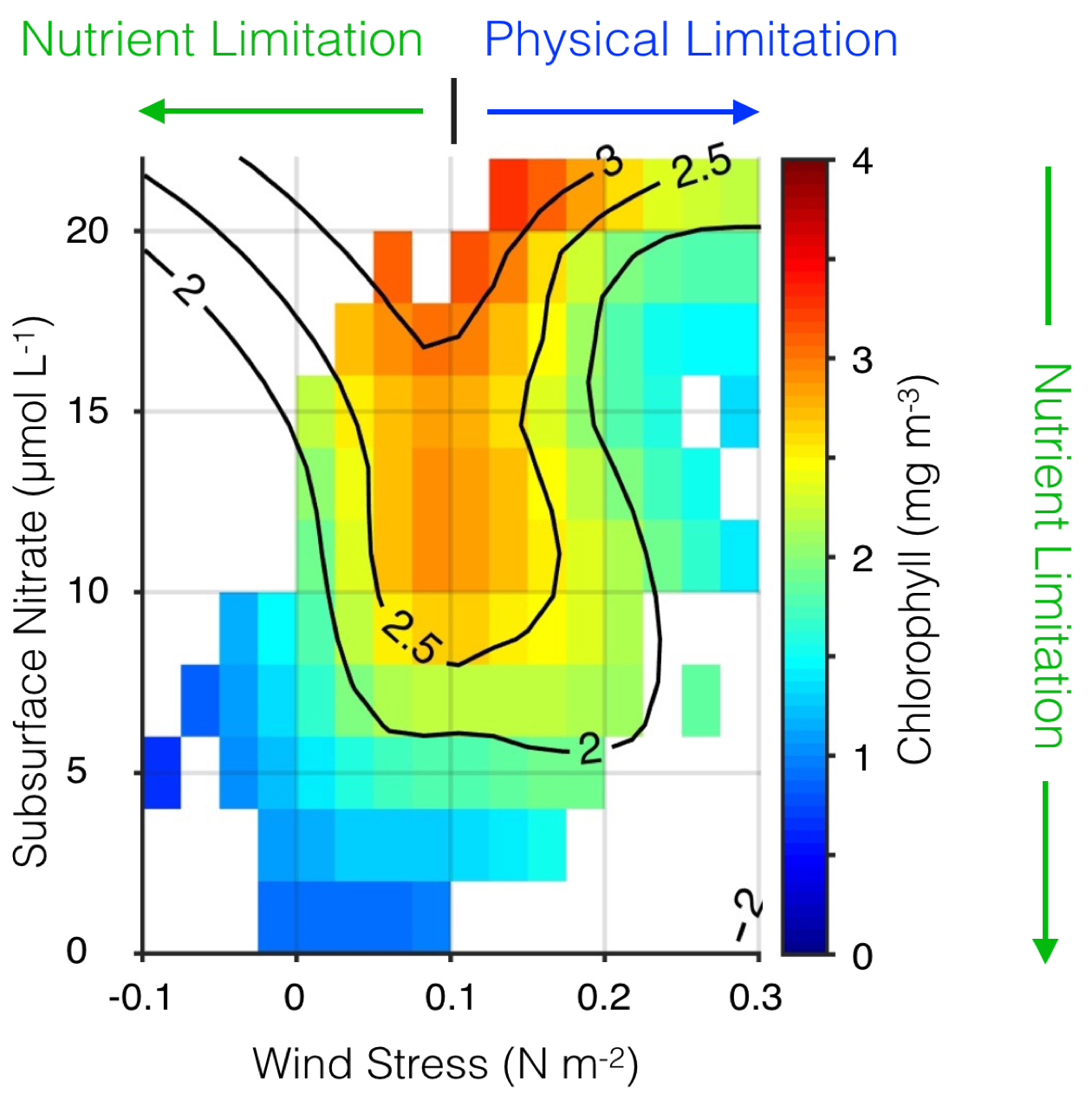
Understanding how fluctuations in the oceans’ turbulence landscape impacts phytoplankton is of fundamental importance, especially for predicting species succession and community structure given projected climate-driven changes in temperature, winds, and upper ocean structure.

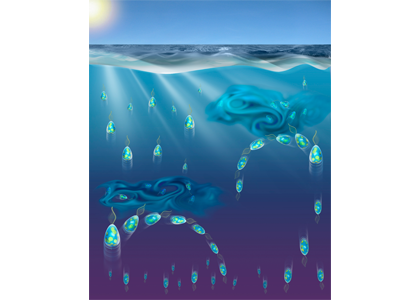
An upward-swimming phytoplankton population splits into upward- and downward-swimming sub-populations when exposed to turbulent eddies, due to a subtle change in cell shape. Illustration by: A. Sengupta, G. Gorick, F. Carrara and R. Stocker
This work was co-funded by a Human Frontier Science Program Cross Disciplinary Fellowship (LT000993/2014-C to A.S.), a Swiss National Science Foundation Early Postdoc Mobility Fellowship (to F.C.), and a Gordon and Betty Moore Marine Microbial Initiative Investigator Award (GBMF 3783 to R.S.)