Trace elements are essential micronutrients for life in the ocean and also serve as valuable fingerprints of chemical weathering. The behaviour of trace elements in the ocean has gained interest because some of these elements are found at vanishingly low concentrations that limit ecosystem productivity. Despite delivering >2000 km3 yr-1 of freshwater to the polar oceans, ice sheets have largely been overlooked as major trace element sources. This is partly due to a lack of data on meltwater endmember chemistry beneath and emerging from the Greenland and Antarctic ice sheets, which cover 10% of Earth’s land surface area, and partly because meltwaters were previously assumed to be dilute compared to most river waters.
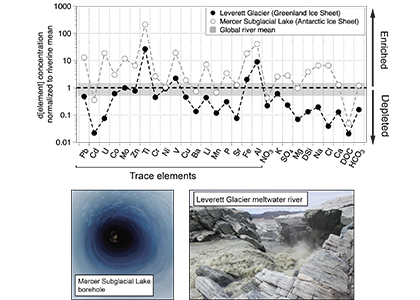
In a study published in PNAS, authors analysed the trace element composition of meltwaters from the Mercer Subglacial Lake, a hydrologically active subglacial lake >1000 m below the surface of the Antarctic Ice Sheet, and a meltwater river emerging from beneath a large outlet glacier of the Greenland Ice Sheet (Leverett Glacier). These subglacial meltwaters (i.e., water travelling along the ice-rock interface beneath an ice mass) contained much higher concentrations of trace elements than anticipated. For example, typically immobile elements like iron and aluminium were observed in the dissolved phase (<0.45 µm) at much higher concentrations than in mean river or open ocean waters (up to 20,900 nM for Fe and 69,100 nM for Al), but exhibited large size fractionation between colloidal/nanoparticulate (0.02 – 0.45 µm) and soluble (<0.02 µm) size fractions (Figure 1). Subglacial physical and biogeochemical weathering processes are thought to mobilize many of these trace elements from the bedrock and sediments beneath ice sheets and export them downstream. Antarctic subglacial meltwaters were more enriched in dissolved trace elements than Greenland Ice Sheet outflow, which is likely due to longer subglacial residence times, lack of dilution from surface meltwater inputs, and differences in underlying sediment geology.
These results indicate that ice sheet systems can mobilize large quantities of trace elements from the land to the ocean and serve as major contributors to regional elemental cycles (e.g., coastal Southern Ocean). In a warming climate with increasing ice sheet runoff, subglacial meltwaters will become an increasingly dynamic source of micronutrients to coastal oceanic ecosystems in the polar regions.
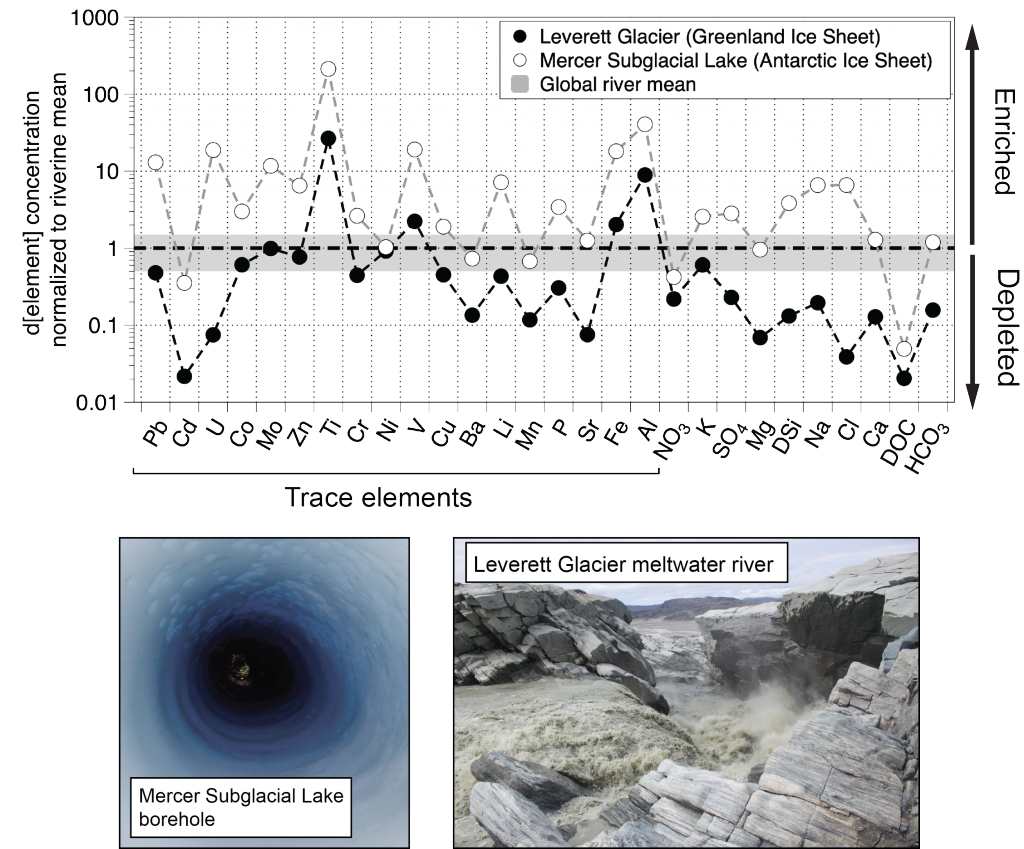
Figure caption: Leverett Glacier (Greenland Ice Sheet) and Mercer Subglacial Lake (Antarctic Ice Sheet) dissolved elemental concentrations (<0.45 µm) normalized to mean non-glacial riverine trace element concentrations (Gaillardet et al., 2014) and major element concentrations (Martin and Meybeck, 1979). Grey regions indicate ±50 % of the riverine mean. Although major elements can be significantly depleted compared to non-glacial rivers, trace elements are commonly similar to or enriched.
Authors:
Jon R. Hawkings (Florida State Univ and German Research Centre for Geosciences)
Mark L. Skidmore (Montana State Univ)
Jemma L. Wadham (Univ of Bristol, UK)
John C. Priscu (Montana State Univ)
Peter L. Morton (Florida State Univ)
Jade E. Hatton (Univ of Bristol, UK)
Christopher B. Gardner (Ohio State Univ)
Tyler J. Kohler (École Polytechnique Fédérale de Lausanne, Switzerland)
Marek Stibal (Charles University, Prague, Czech Republic)
Elizabeth A. Bagshaw (Cardiff Univ, UK)
August Steigmeyer (Montana State Univ)
Joel Barker (Univ of Minnesota)
John E. Dore (Montana State Univ)
W. Berry Lyons (Ohio State Univ)
Martyn Tranter (Univ of Bristol, UK)
Robert G. M. Spencer (Florida State Univ)
SALSA Science Team