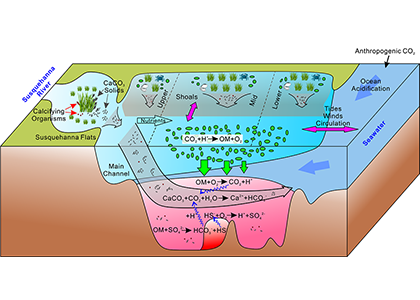
Ocean acidification is often enhanced by eutrophication and subsequent hypoxia and anoxia in coastal waters, which collectively threaten marine organisms and ecosystems. Acidification is particularly of concern for organisms that form shells and skeletons from calcium carbonate (CaCO3) such as commercially important shellfish species. Given that CaCO3 mineral dissolution can increase the total alkalinity of water and neutralize anthropogenic and metabolic CO2, it is important to include CaCO3 cycle in the coastal water acidification study. However, very few studies have linked CaCO3 dissolution to the timing and location of its formation in coastal waters. A recent study from the Chesapeake Bay published in Nature Geoscience reveals the occurrence of a bay-wide pH-buffering mechanism resulting from spatially decoupled CaCO3 mineral cycling (Figure 1). Photosynthesis by submerged aquatic vegetation at the head of the Bay and in other shallow, nearshore waters can remove nutrient pollution from the Bay, generate very high pH, and elevate the carbonate mineral saturation state (Figure 1). This facilitates the formation of CaCO3 minerals, which are then transported downstream along with other biologically produced carbonate shells into acidic subsurface waters, where they dissolve. This dissolution of carbonate minerals helps “buffer” the water against pH decreases and even drive pH increases. This finding has great ecological and natural resource management significance, in that coastal nutrient management and reduction via the resurgence of submerged aquatic vegetation can help mitigate low oxygen and acidification stress for these environments and organisms.
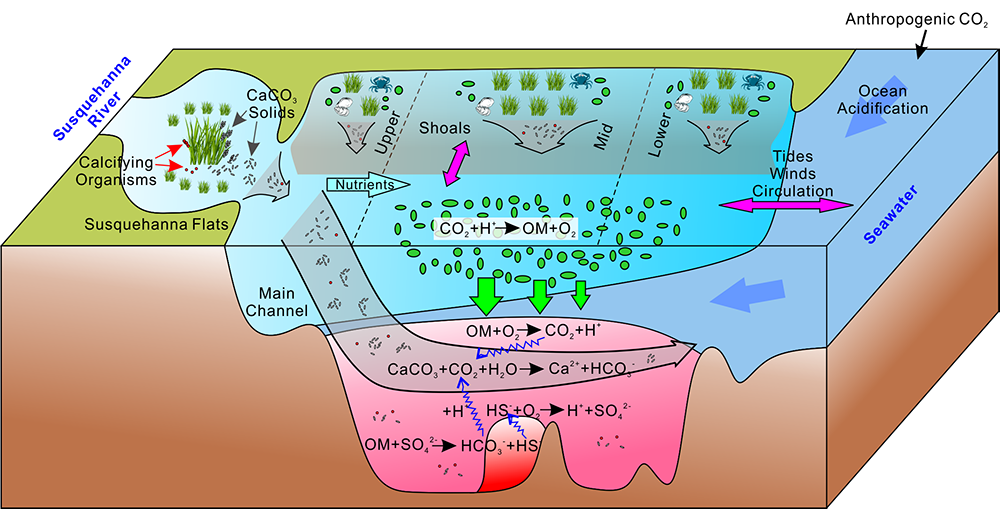
Figure 1: Conceptual model of the self-regulated pH-buffering mechanism in the Chesapeake Bay. Calcium carbonate is formed within the high-pH submerged aquatic vegetation beds in shallow waters (top left and upper part of diagram, all Shoals with SAV), where it could be subsequently transported longitudinally and/or laterally into the deep main channel of the mid and lower bay (center) and upon dissolution, increase pH buffering capacity and alleviate coastal acidification (lower section).
Authors:
Jianzhong Su (University of Delaware, Xiamen University)
Wei-Jun Cai (University of Delaware)
Jean Brodeur (University of Delaware)
Baoshan Chen (University of Delaware)
Najid Hussain (University of Delaware)
Yichen Yao (University of Delaware)
Chaoying Ni (University of Delaware)
Jeremy Testa (University of Maryland Center for Environmental Science)
Ming Li (University of Maryland Center for Environmental Science)
Xiaohui Xie (University of Maryland Center for Environmental Science, Second Institute of Oceanography)
Wenfei Ni (University of Maryland Center for Environmental Science)
K. Michael Scaboo (University of Delaware)
Yuanyuan Xu (University of Delaware)
Jeffrey Cornwell (University of Maryland Center for Environmental Science)
Cassie Gurbisz (St. Mary’s College of Maryland)
Michael S. Owens (University of Maryland Center for Environmental Science)
George G. Waldbusser (Oregon State University)
Minhan Dai (Xiamen University)
W. Michael Kemp (University of Maryland Center for Environmental Science)