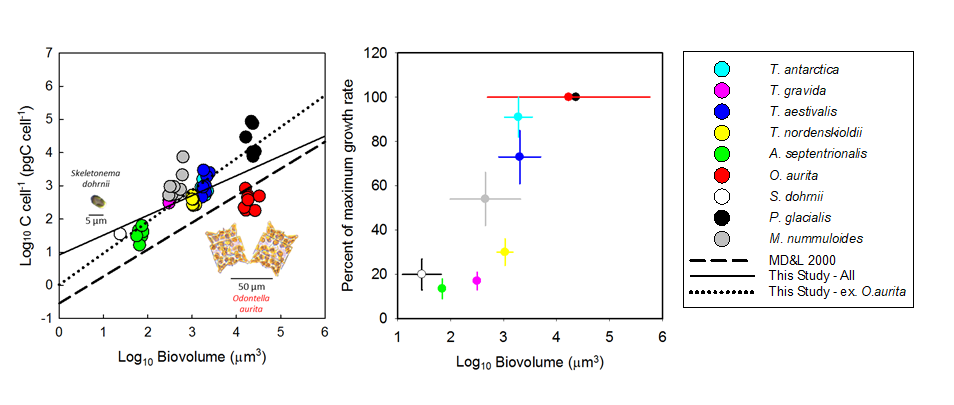
Oceanic dissolved organic carbon (DOC) ultimately exchanges with atmospheric CO2 and thus represents an important carbon source/sink with consequence for climate. Most of the DOC is recalcitrant to microbial degradation, with some fractions surviving for thousands of years. Therefore, DOC in the deep ocean was thought to be stable or to decrease slowly over decades to centuries due to biotic and abiotic sinks. However, a study published in Global Biogeochemical Cycles shows that there are some zones of the deep Atlantic Ocean where recalcitrant DOC experiences net production. Using data from oceanographic cruises across the Atlantic Ocean, the authors first identified the major water masses in the basin and the percentage of each in every sample taken for DOC analysis. The study revealed net additions of 27 million tons of dissolved organic carbon per year in the deep South Atlantic. On the other hand, the North Atlantic serves as a net sink, removing 298 million tons of carbon annually. DOC production observed in the deep Atlantic is probably due to the sinking particles that solubilize into DOC, since DOC enrichment was most evident at latitudes characterized as elevated productivity divergence zones.
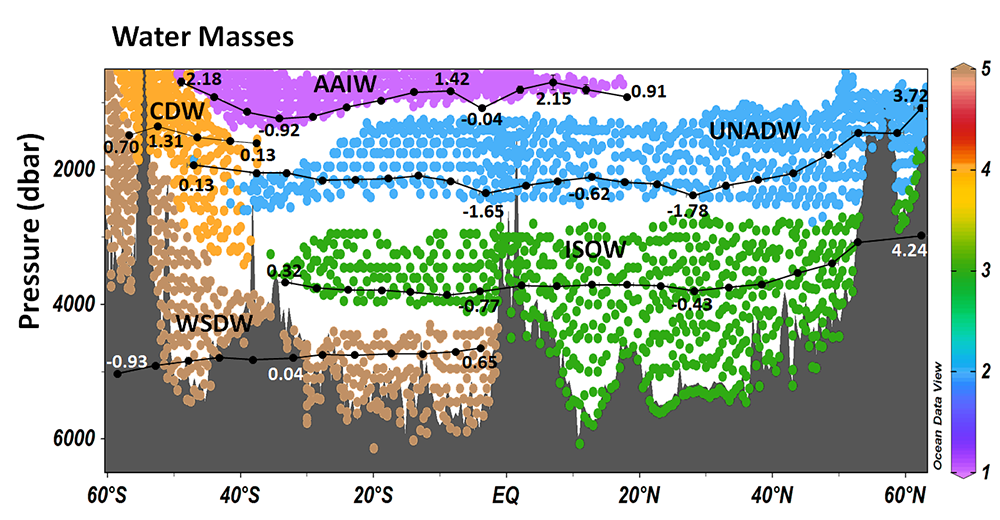
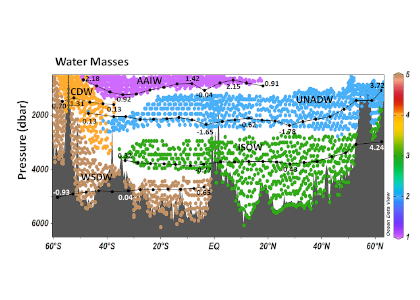
Figure 1. Water masses along GO-SHIP line A16 (colored dots) and recalcitrant DOC variations due to biogeochemical processes (black dots within each water mass) in the deep Atlantic Ocean. Water mass domains are defined as the set of samples with the corresponding water mass proportion ≥50%. Recalcitrant DOC latitudinal variations per water stratum due to biogeochemical processes (ΔDOC) is in μmol kg-1. Numbers on the plots are DOC values for the corresponding dots. Scales (not shown) are the same for all the plots, from -4 to 6 μmol kg-1. Positive (negative) ΔDOC indicates values higher (lower) than the average DOC calculated for each water mass using an optimum multiparameter (OMP) analysis. DOC = dissolved organic carbon. AAIW = Antarctic Intermediate Water; UNADW = upper North Atlantic Deep Water; ISOW = Iceland Scotland Overflow Water; CDW = Circumpolar Deep Water; WSDW = Weddell Sea Deep Water. Figure created with Ocean Data View (Schlitzer, 2015).
Considering that the net DOC production over the entire Atlantic basin euphotic zone is 0.70–0.75 Pg C year-1, the authors estimated that 30–39% of that DOC is consumed in the deep Atlantic subsequent to its export by overturning circulation. The upper North Atlantic Deep Water (UNADW) acts as the primary sink, accounting for 66% of the recalcitrant DOC removal in the North Atlantic. Conversely, the Antarctic Intermediate Water (AAIW) is the primary recipient, with 45% of recalcitrant DOC production in the South Atlantic, closely followed by the old UNADW that gains 44% of the recalcitrant DOC in the southern basin.
The Atlantic works as a mosaic of water masses, where both removal and addition of recalcitrant DOC occurs, with the dominant term dependent on the origin, temperature, age and depth of the water masses. The production of recalcitrant DOC in the deep ocean should be considered in biogeochemical models dealing with the carbon cycle and climate.
Authors:
C. Romera-Castillo and J. L. Pelegrí (Instituto de Ciencias del Mar, CSIC, Spain)
M. Álvarez (Instituto Español de Oceanografía, Spain)
D. A. Hansell (University of Miami, USA)
X. A. Álvarez-Salgado (Instituto de Investigaciones Marinas, CSIC, Spain)