How does the enormous diversity of zooplankton species, life cycles, size, feeding ecology, and physiology affect their role in ocean food webs and cycling of carbon?
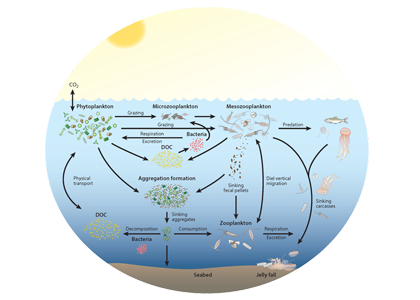
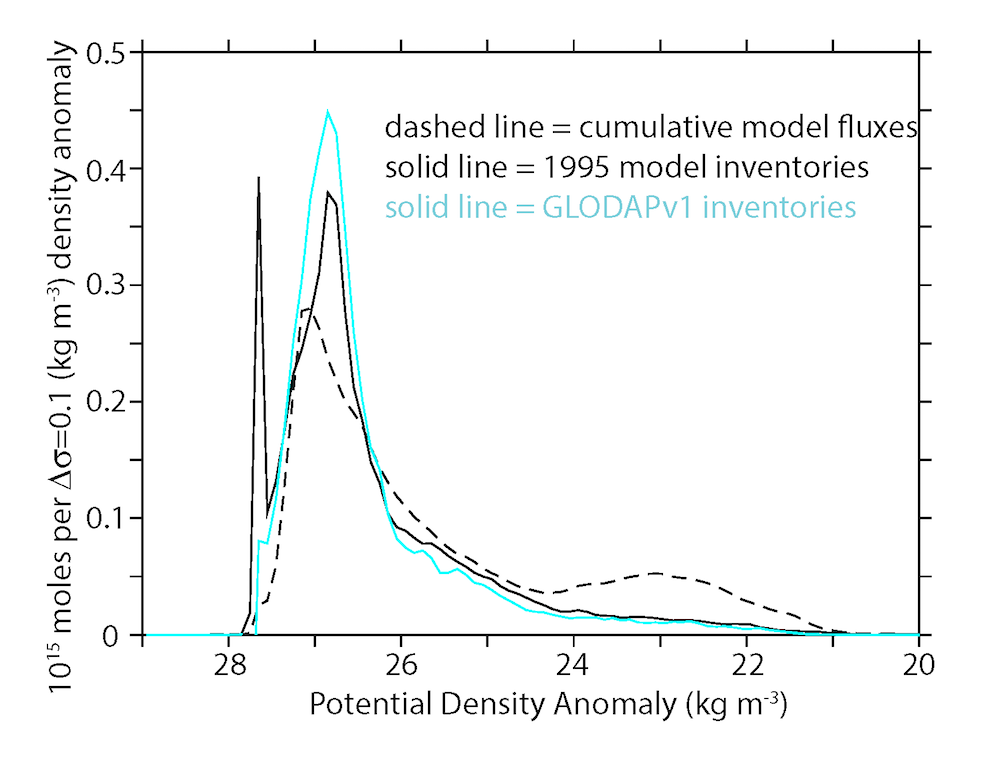
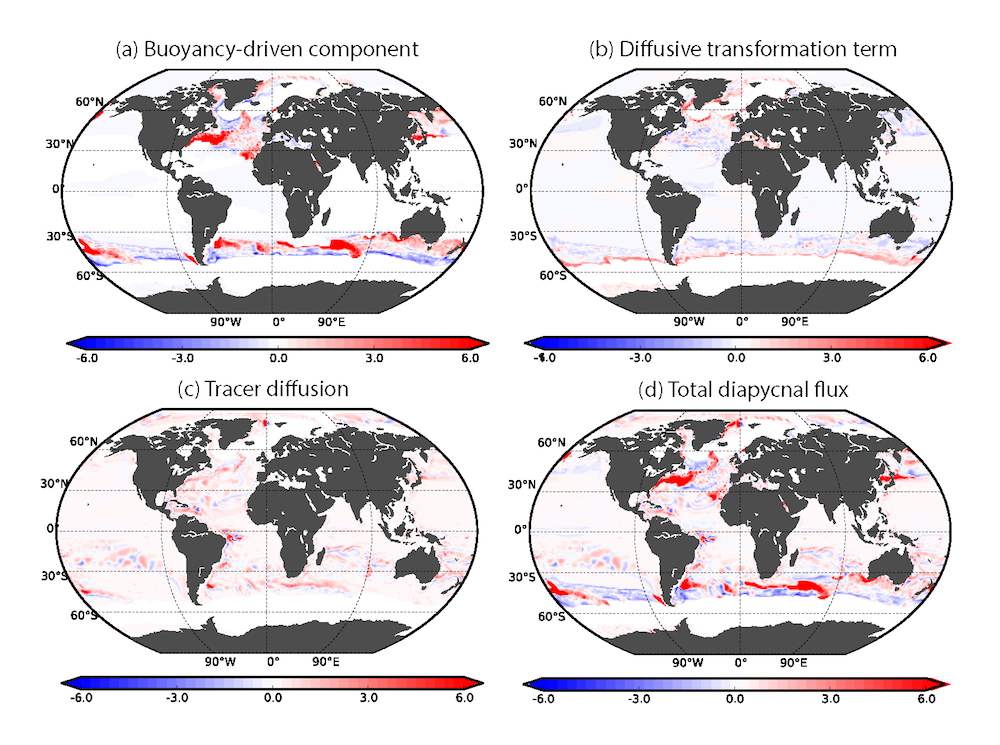
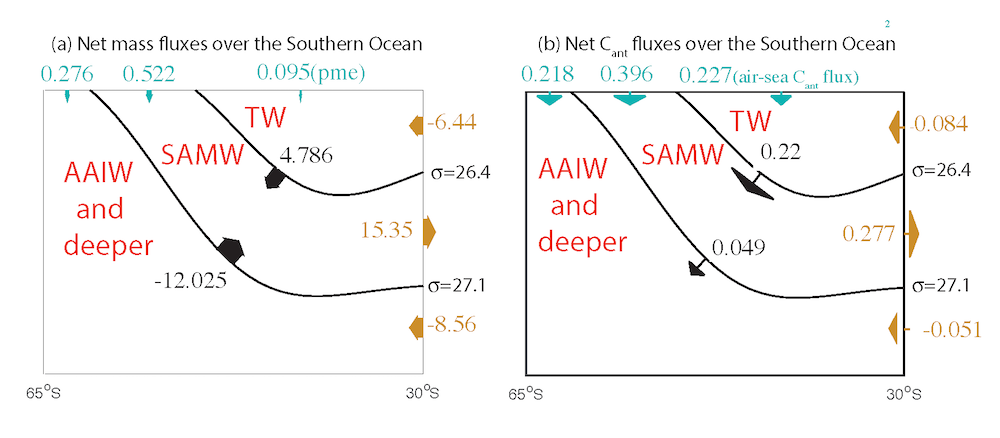
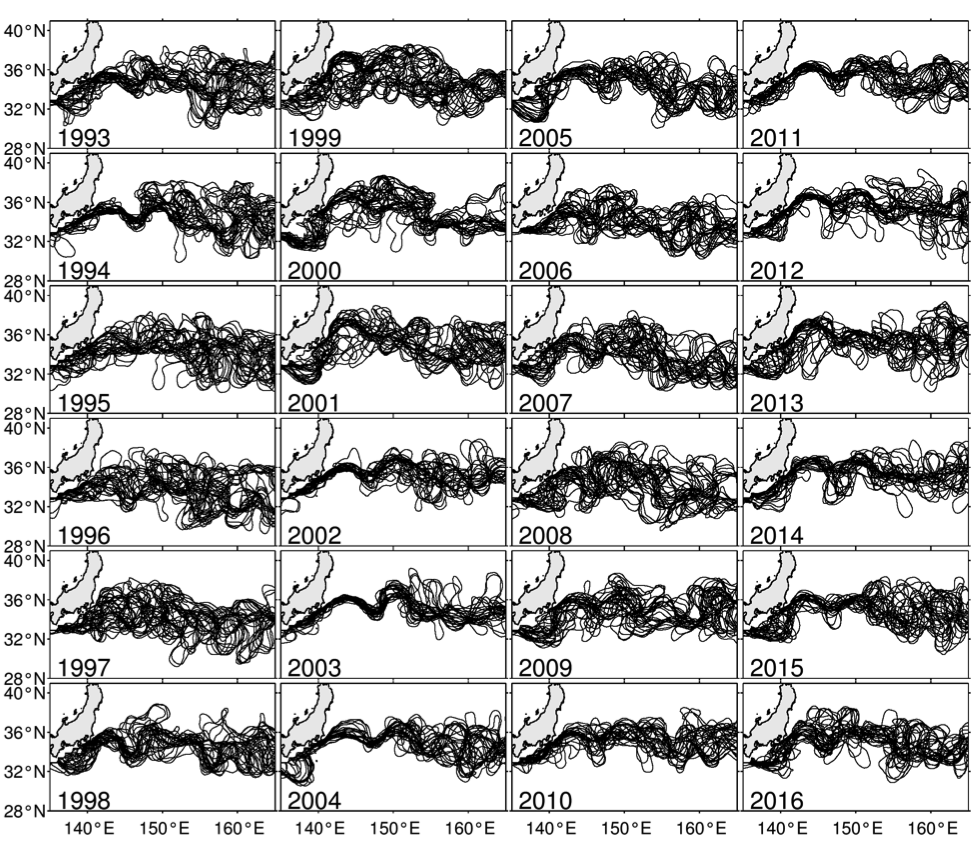
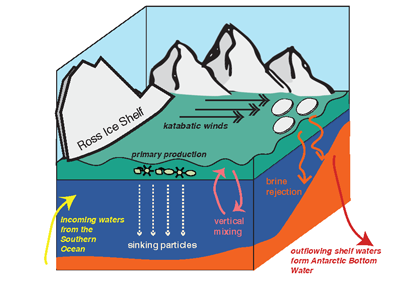
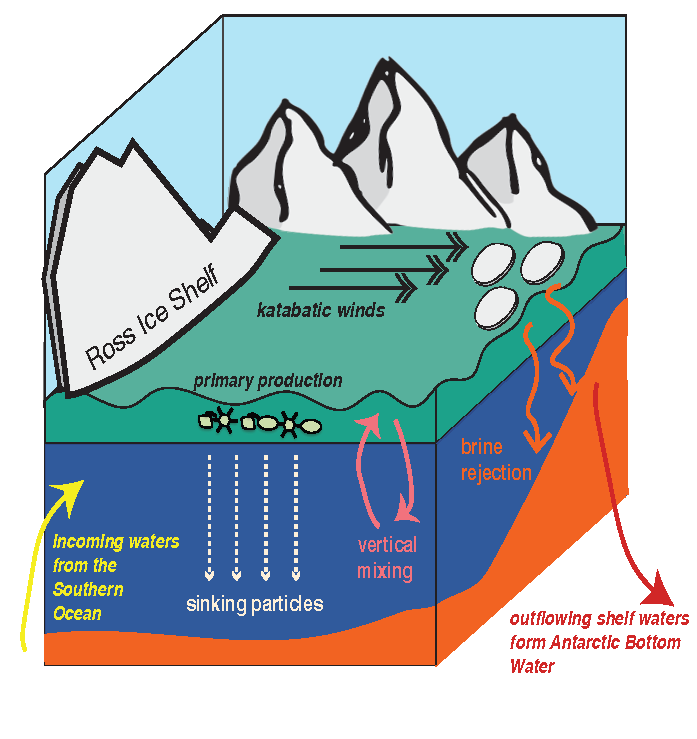
In the 2017 issue of Annual Review of Marine Science, Steinberg and Landry review the fundamental and multifaceted roles that zooplankton play in the cycling and export of carbon in the ocean. Carbon flows through marine pelagic ecosystems are complex due to the diversity of zooplankton consumers and the many trophic levels they occupy in the food web–from single-celled herbivores to large carnivorous jellyfish. Zooplankton also contribute to carbon export processes through a variety of mechanisms (mucous feeding webs, fecal pellets, molts, carcasses, and vertical migrations).
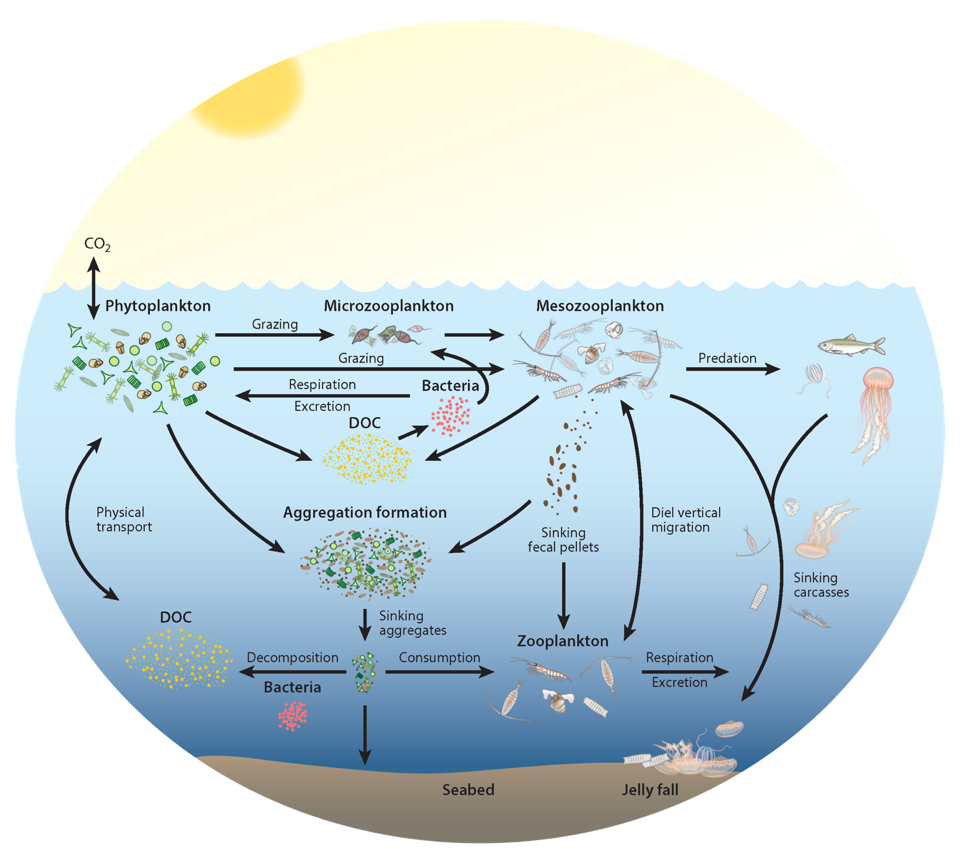
Figure 1. Pathways of cycling and export of carbon by zooplankton in the ocean.
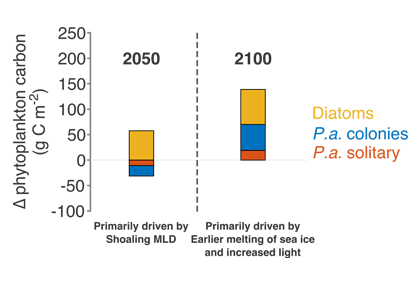
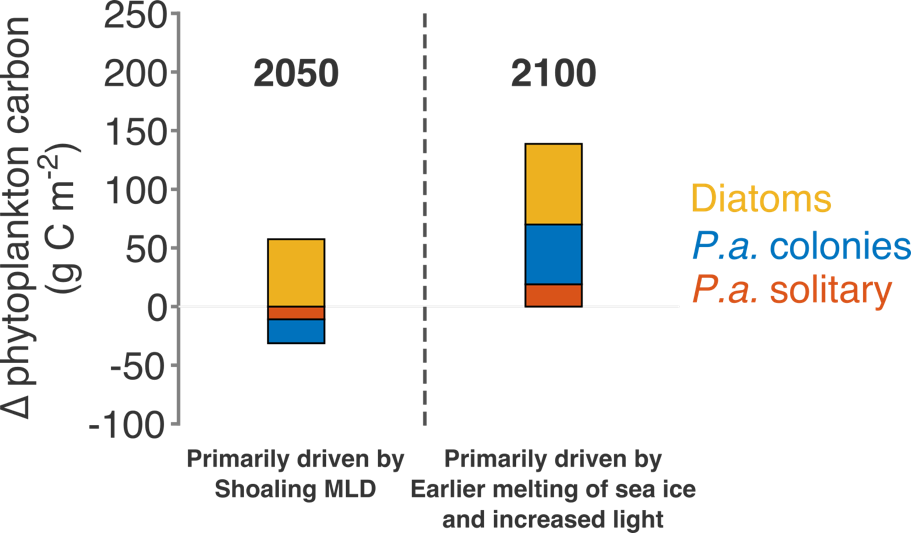
Climate change and other stressors are already affecting zooplankton abundance, distribution, and life cycles, and are predicted to result in widespread changes in zooplankton carbon cycling in the future. These changes will affect both the larger marine food web that depends upon zooplankton for food (fish) or recycled products for growth (primary producers) and the amount of carbon exported into the deep sea–where far from contact with the atmosphere it no longer contributes to global warming.
Authors:
Deborah K. Steinberg, Virginia Institute of Marine Science, The College of William and Mary
Michael R. Landry, Scripps Institution of Oceanography