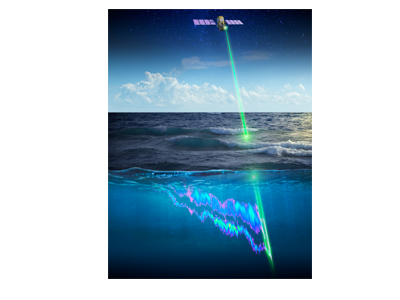
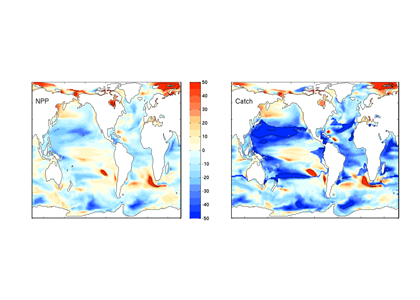
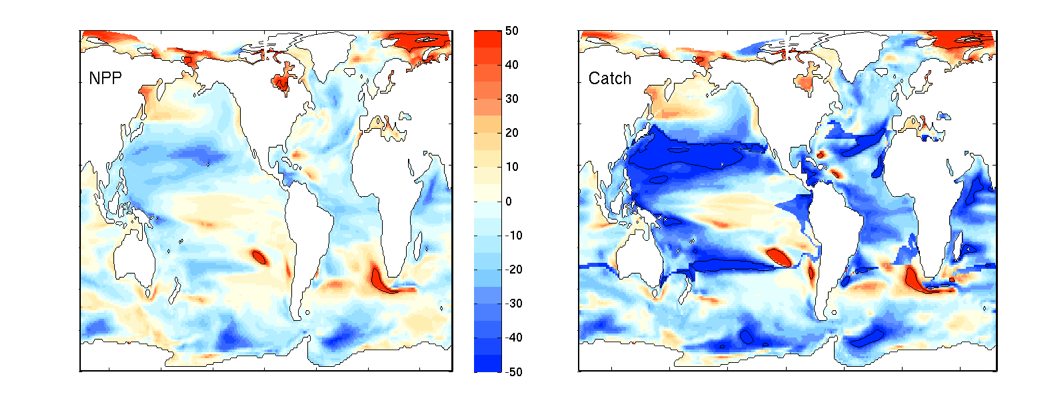
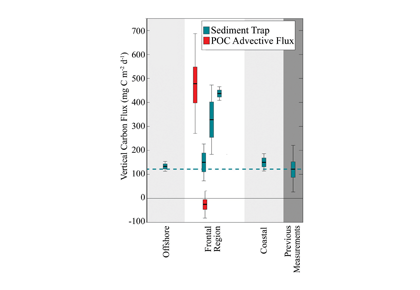
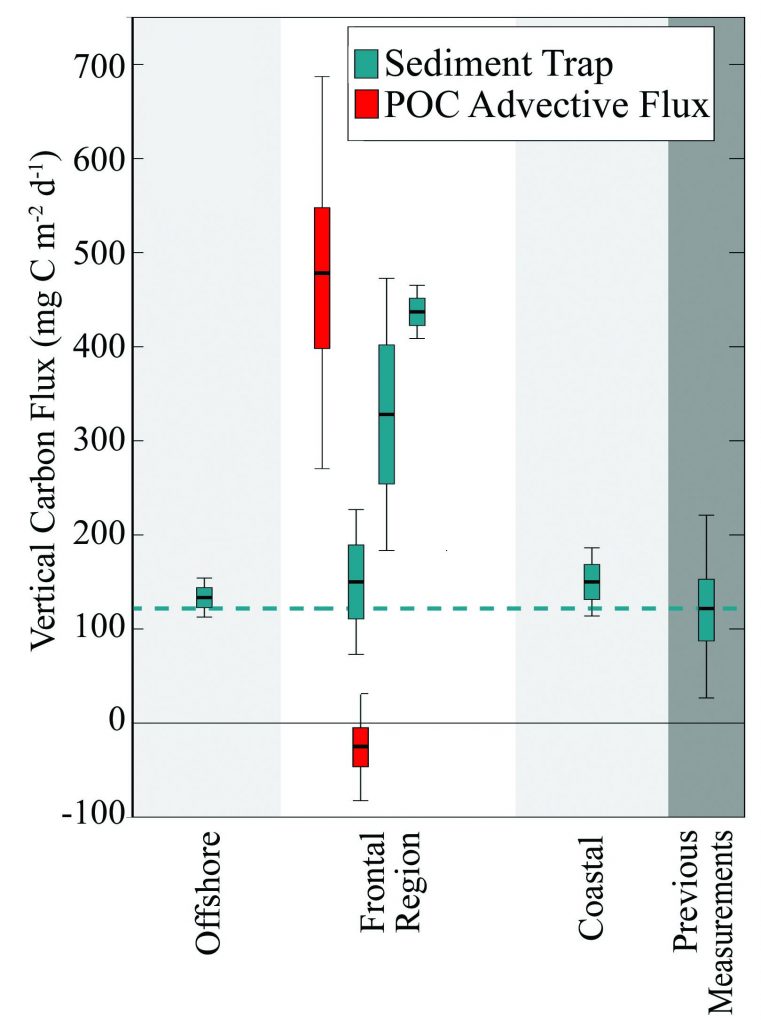
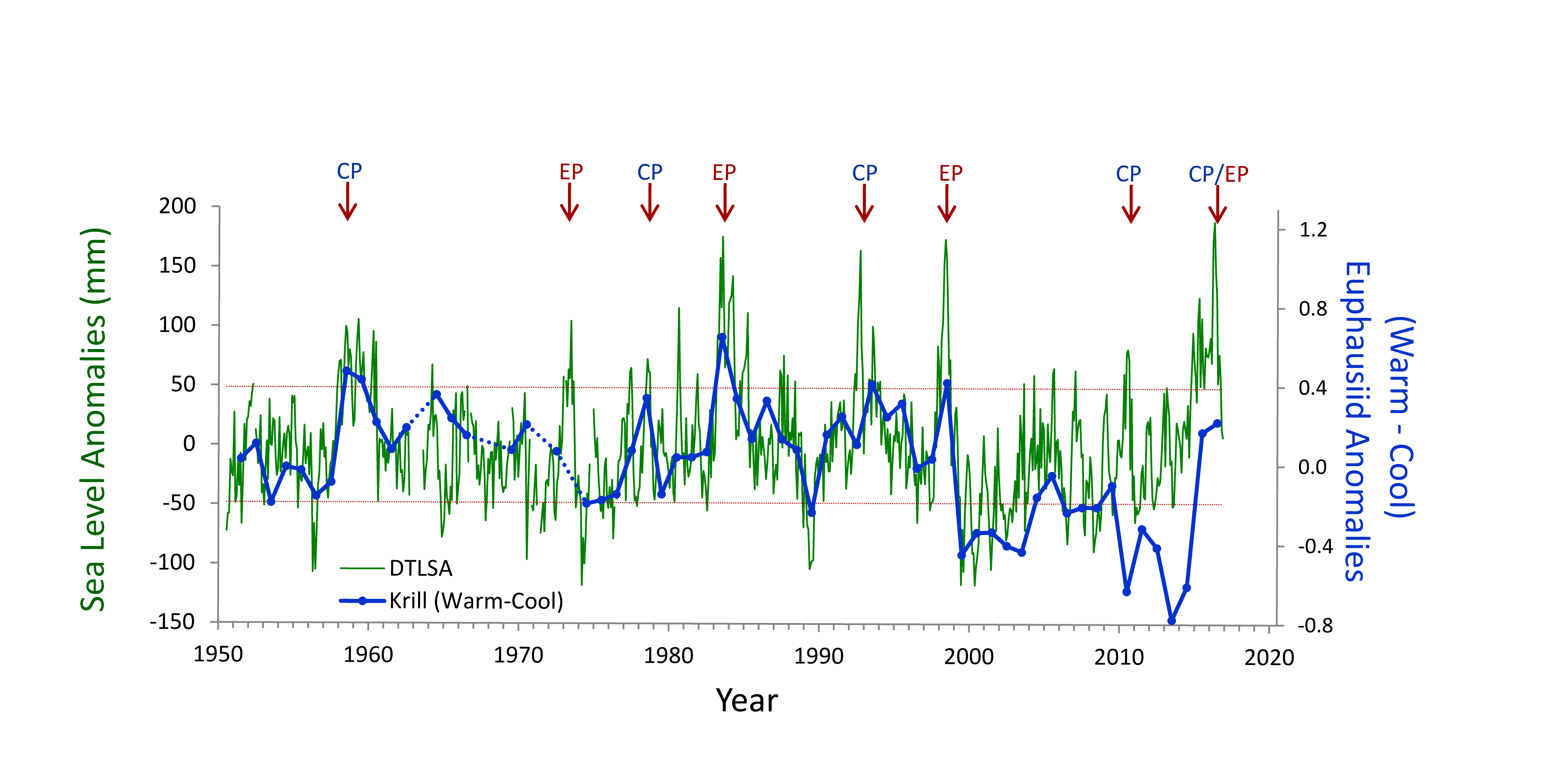
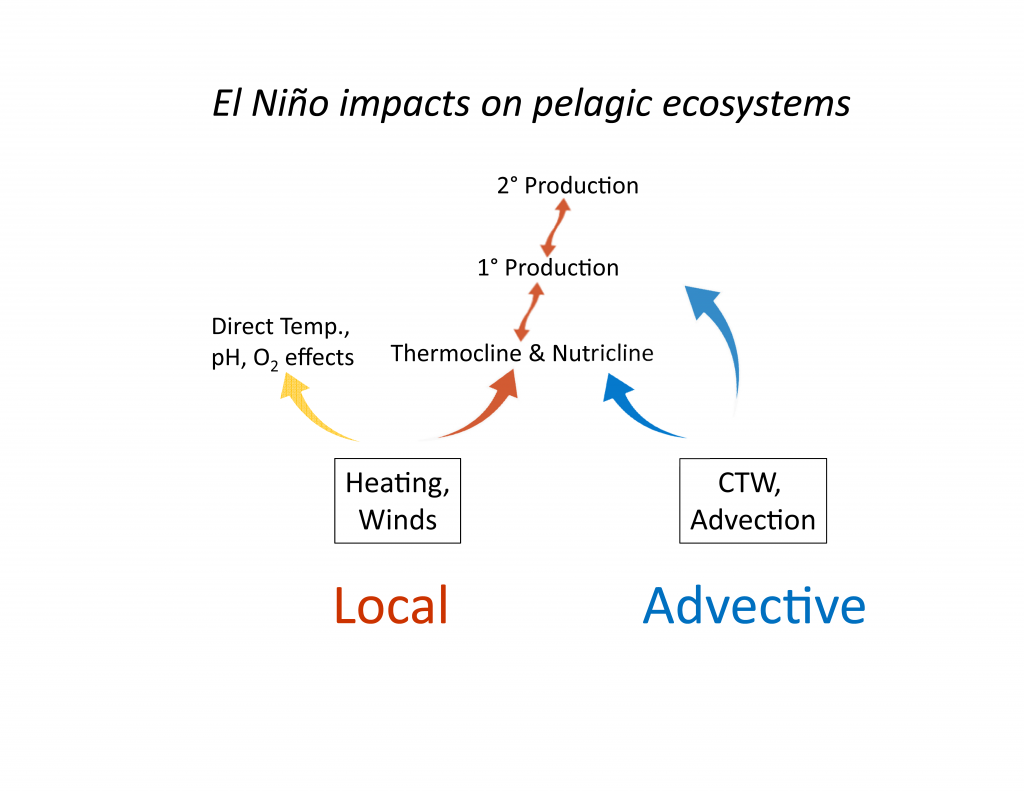
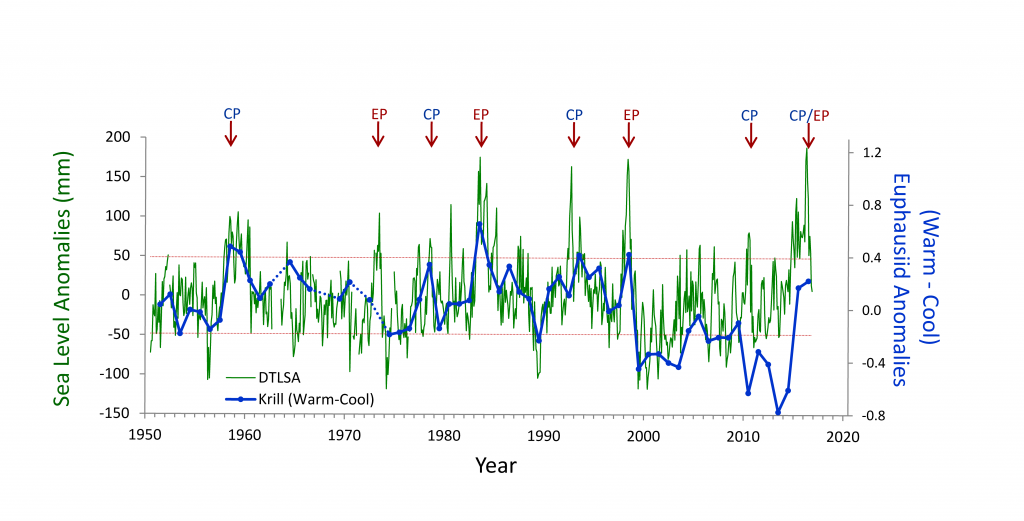
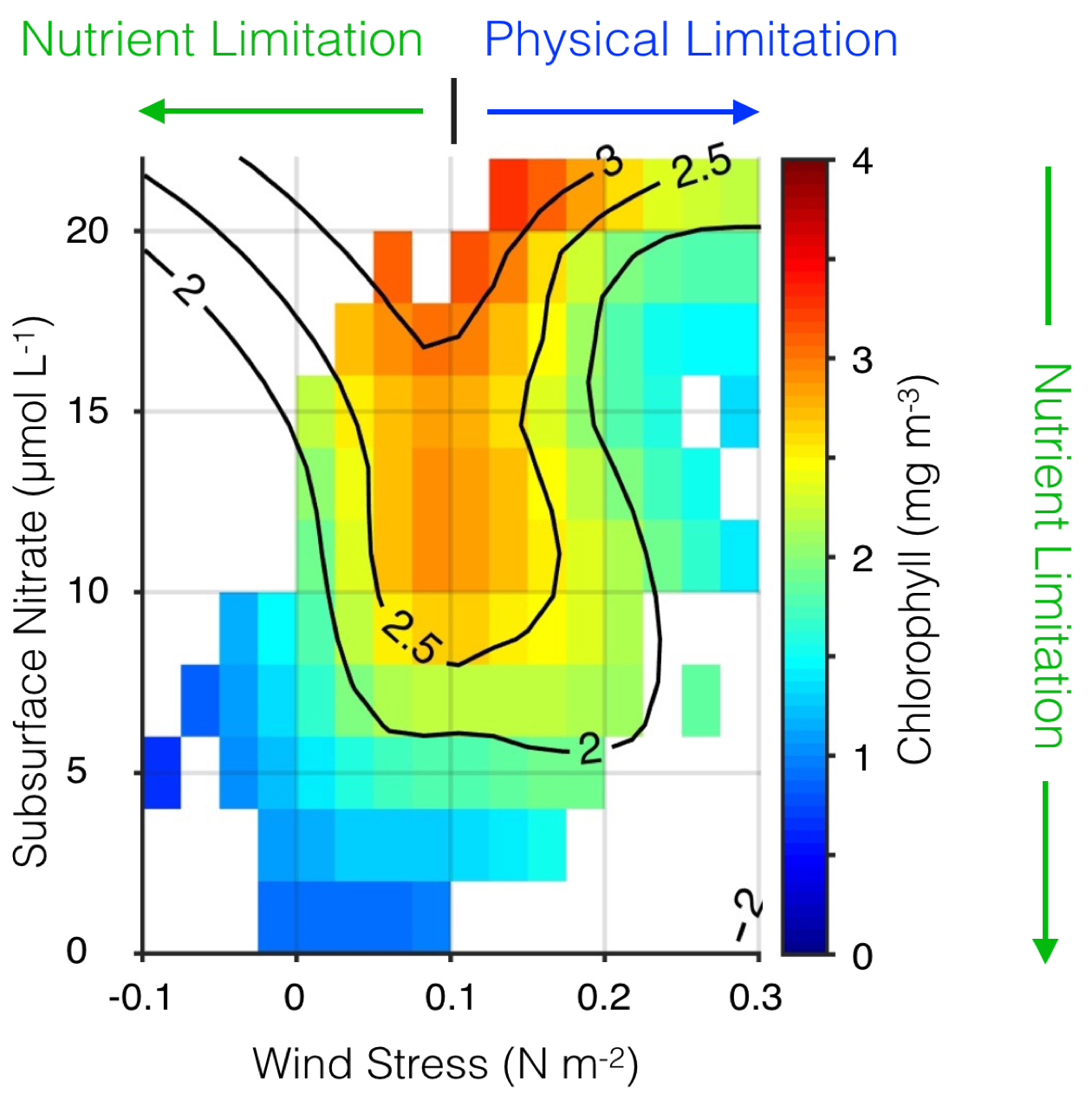
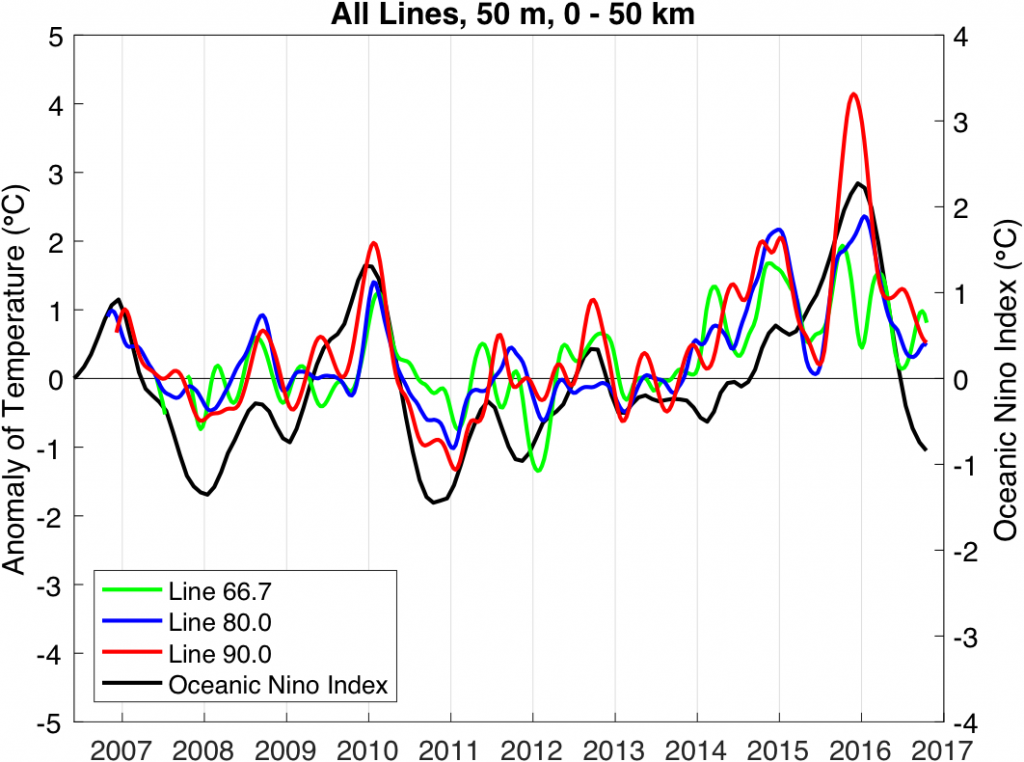
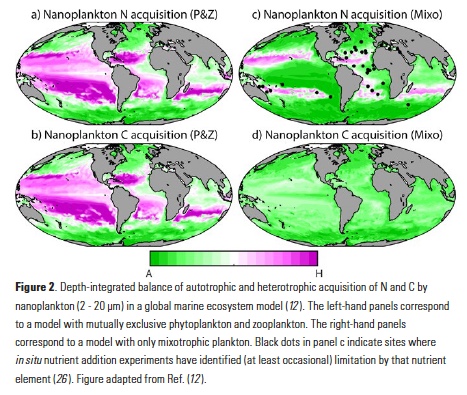
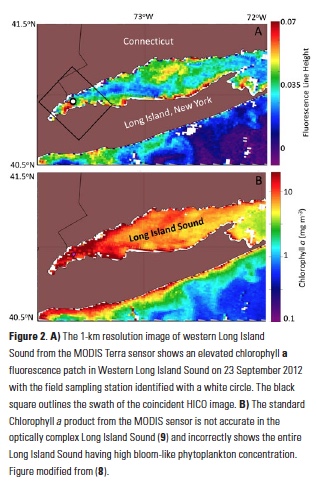
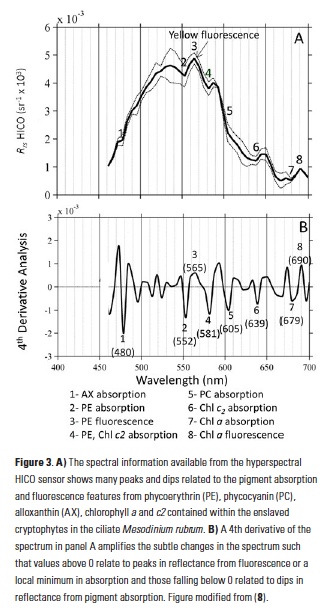
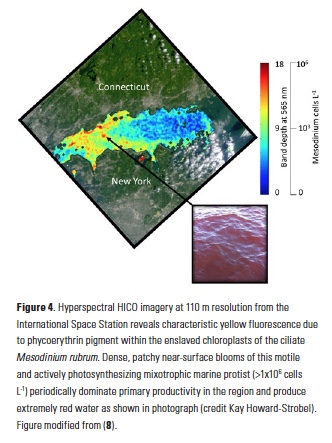
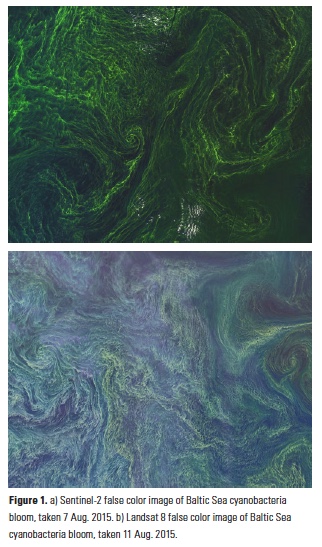
What controls annual cycles and interannual changes in polar phytoplankton biomass? Answers to this question are now emerging from a satellite light detection and ranging (lidar) sensor, which can observe the polar oceans throughout the extensive periods when measurements from traditional passive ocean color sensors are impossible. The new study uses active lidar measurements from the CALIOP satellite sensor to construct complete decade-long record of phytoplankton biomass in the northern and southern polar regions. Results of the study show that annual cycles in biomass are driven by rates of acceleration and deceleration in phytoplankton division, with bloom termination coinciding with maximum division rates irrespective of whether nutrients are exhausted. The study further shows that interannual differences in bloom strength can be quantitatively related to the difference between the winter minimum to summer maximum in division rates. Finally, the analysis indicated that ecological processes had a greater impact than ice cover changes on integrated polar zone phytoplankton biomass in the north, whereas ice cover changes were the dominant driver in the south polar zone. Despite being designed for atmospheric research, CALIOP has provided the first demonstration that active satellite lidar measurements can yield important new insights on plankton ecology in the climate sensitive polar regions. This proof-of-concept creates a foundation for a future ocean-optimized sensor with water-column profiling capabilities that would launch a new lidar era in satellite oceanography.

Authors:
Michael J. Behrenfeld (Oregon State Univ.)
Yongxiang Hu (NASA Langley Research Center)
Robert T. O’Malley (Oregon State Univ.)
Emmanuel S. Boss (Univ. Maine)
Chris A. Hostetler (NASA Langley Research Center)
David A. Siegel (Univ. California Santa Barbara)
Jorge Sarmiento (Princeton Univ.)
Jennifer Schulien (Oregon State Univ.)
Johnathan W. Hair (NASA Langley Research Center)
Xiaomei Lu (NASA Langley Research Center)
Sharon Rodier (NASA Langley Research Center)
Amy Jo Scarino (NASA Langley Research Center)