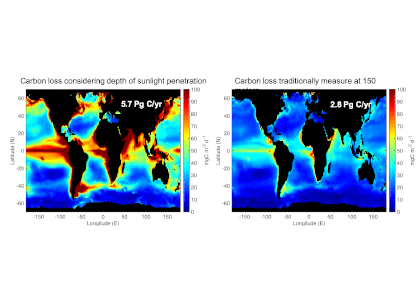
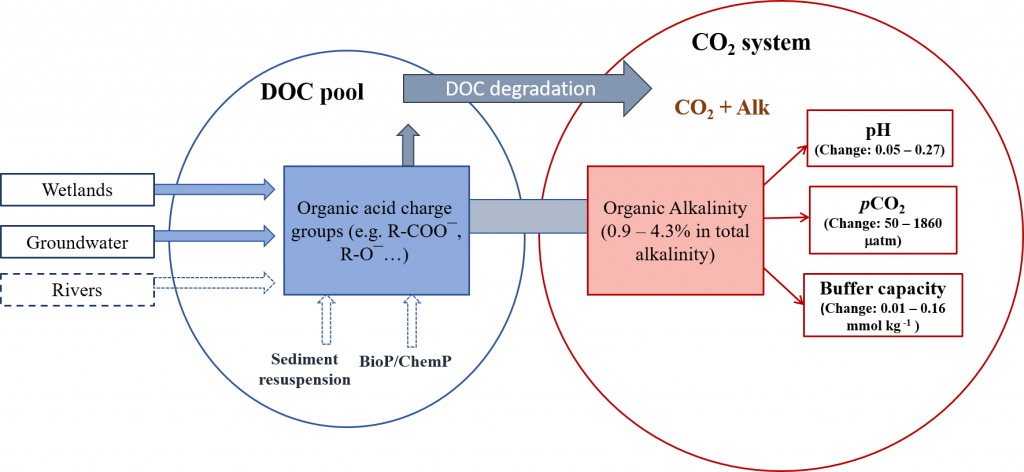
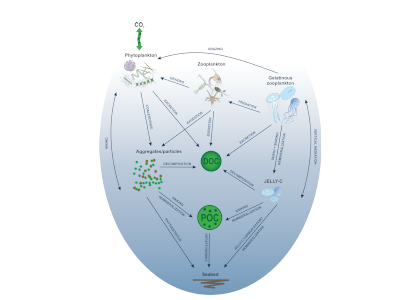
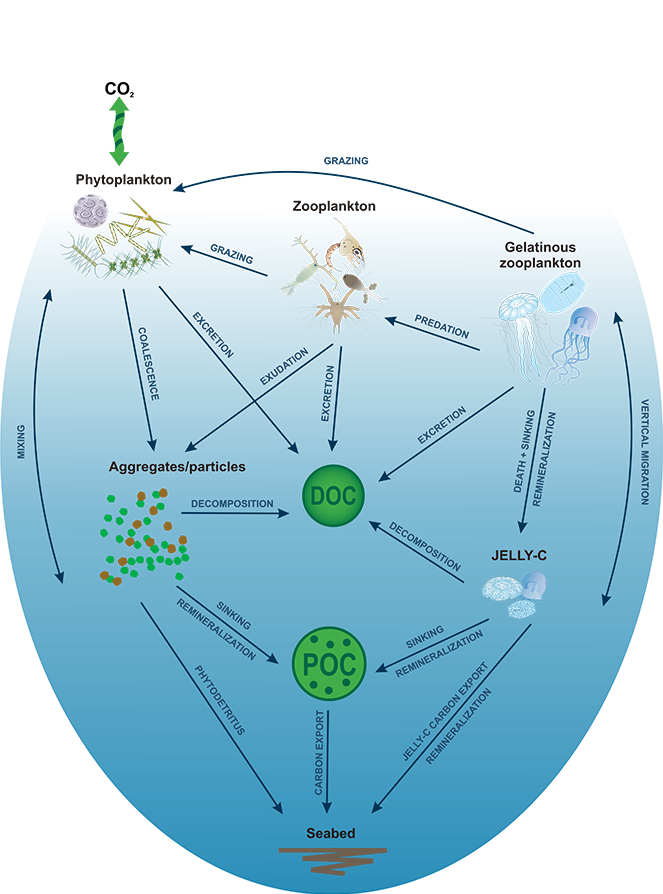
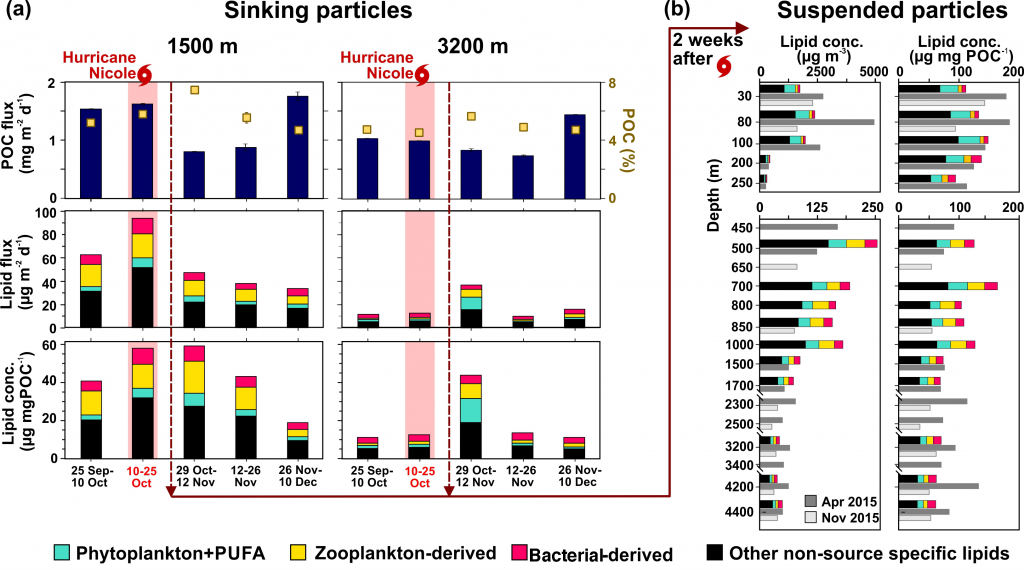
Organic carbon produced during photosynthesis in the sunlit euphotic zone is transported to the deep ocean via the ocean’s biological carbon pump (BCP). Even small changes in the BCP efficiency changes the carbon dioxide gradient across the ocean‐atmosphere interface, thus influencing global climate. A recent study in PNAS demonstrate that prior studies that estimate BCP efficiencies at a fixed depth fail because they do not consider the varying depth of light penetration, which ultimately controls production of sinking organic carbon and varies by location and season. Subsequently, the fixed depth approach introduces regional biases and underestimates global estimates of BCP efficiency by two-fold (Figure 1). These new findings make the case for using euphotic zone‐based metrics rather than applying a fixed depth to compare BCP efficiencies between sites. Improved estimates of BCP efficiency will lead to a better understanding of the mechanisms that control ocean carbon fluxes and its feedbacks on climate.
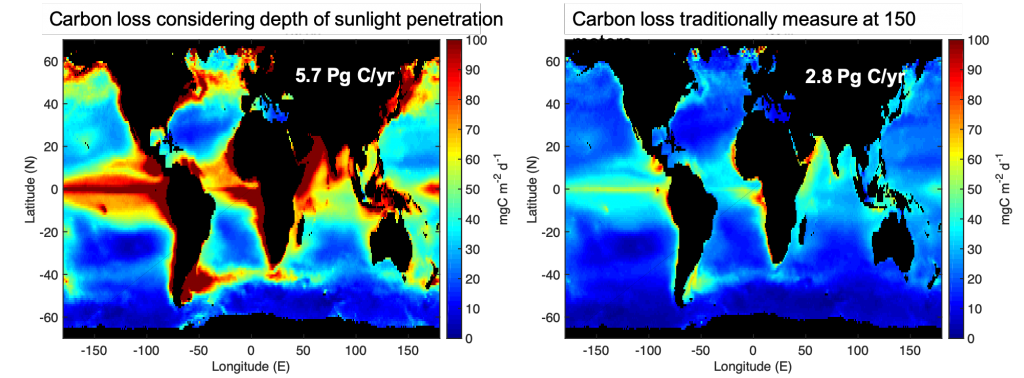
Figure 1: Carbon loss from the surface ocean shows more variability and is twice as high if measured at the depth where sunlight penetrates (left) vs. 150 meters (about 500 feet; right) where it is commonly measured. One Pg is 1015 grams with close to 6 Pg of carbon being transported to depth per year in left panel. In comparison, about 10 Pg C/yr is released to the atmosphere as a result of human activity.
Authors:
Ken Buesseler (WHOI)
Philip Boyd (IMAS Univ. Tasmania)
Erin Black (Dalhousie University)
David Siegel (University of California, Santa Barbara)
Also see: Tiny plankton drive processes in the ocean that capture twice as much carbon as scientists thought on The Conversation.
Featured on the cover of the PNAS May 5, 2020 issue: