Coastal water temperatures have been increasing globally with more frequent marine heat waves threatening marine life and nearshore communities reliant upon these ecosystems. Often, this warming is assumed to be uniform in space and time; however, this is not the case in the Chesapeake Bay, where warming waters play a major role in exacerbating low oxygen levels and indirectly limiting the efficacy of nutrient reduction efforts on land.
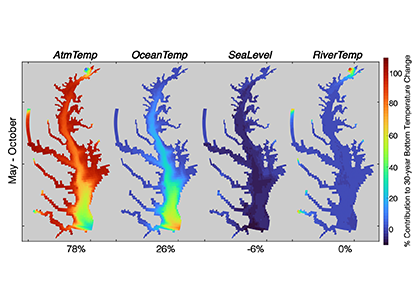
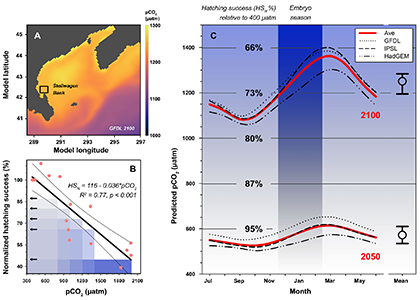
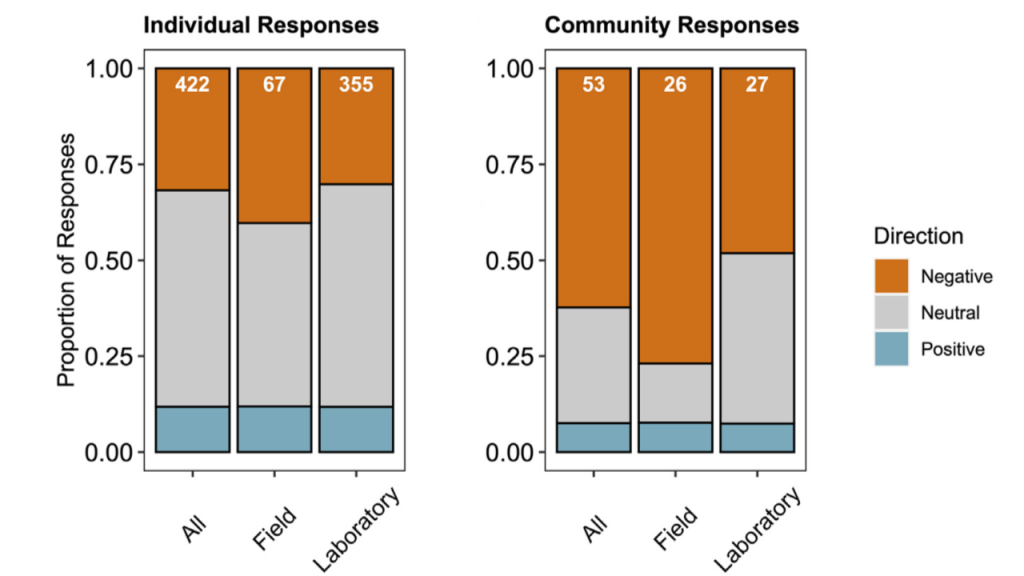
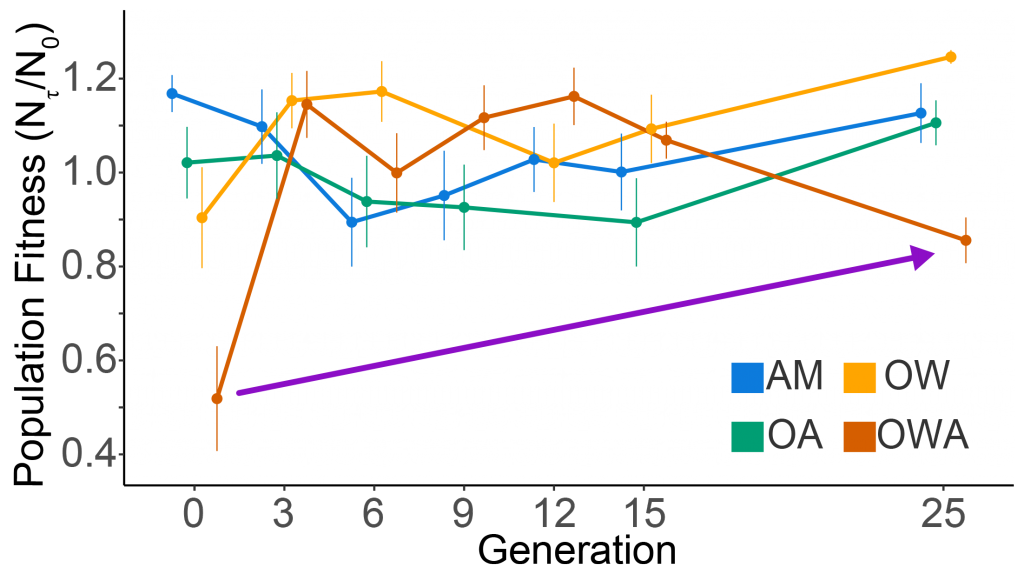
New research published in the Journal of the American Water Resources Association combined long-term observations and a hydrodynamic model to quantify the temporal and spatial variability in warming Chesapeake Bay waters, and identify the contributions of different mechanisms driving these historical temperature changes. While winter temperatures have warmed by less than a half a degree over the past 30 years, summer temperatures have warmed by nearly 1.5 °C, with similar increases at the surface and bottom. In cooler months, the atmosphere was the dominant driver of warming throughout the majority of the Bay, but oceanic warming explained more than half of the increased summer temperatures in the southern Bay nearest the Atlantic.
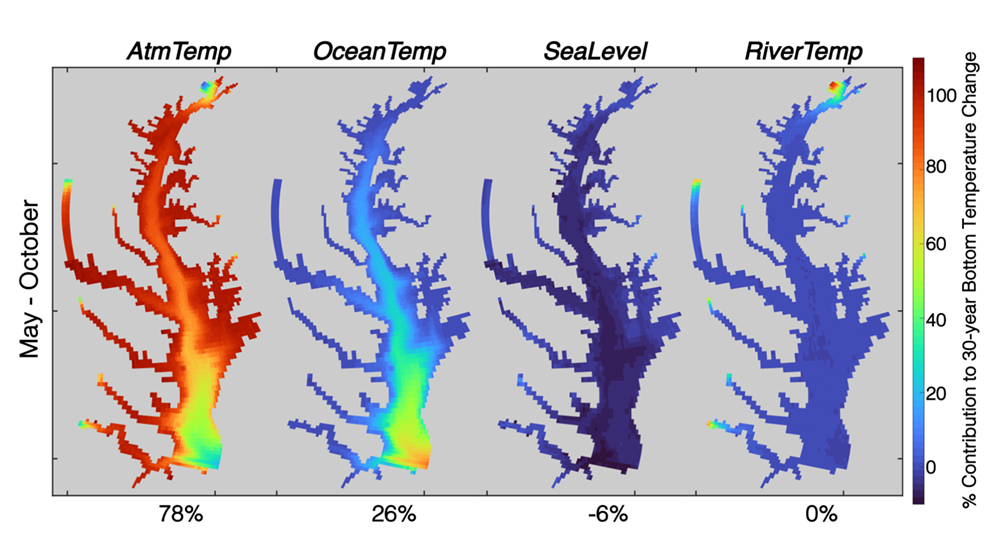
Figure 1: Relative contribution of different factors to warm-month Chesapeake Bay temperature change over the period 1985-2015. Percentages correspond to average main channel contributions for each component.
Warming temperatures have potentially significant implications for the future size of the Chesapeake Bay dead zone, and the marine species directly affected by these low oxygen conditions. Better quantifying warming contributions from the atmosphere, ocean, sea level, and rivers will also help constrain regional temperature projections throughout the estuary. More accurate projections of future Bay temperatures can help coastal managers better understand the potential for invasive species expansion and endemic species loss, impacts to fisheries and aquaculture, and how changes to ecosystem processes may impact coastal communities dependent on a healthy Bay.
Authors:
Kyle E. Hinson (Virginia Institute of Marine Science, William & Mary)
Marjorie A. M. Friedrichs (Virginia Institute of Marine Science, William & Mary)
Pierre St-Laurent (Virginia Institute of Marine Science, William & Mary)
Fei Da (Virginia Institute of Marine Science, William & Mary)
Raymond G. Najjar (The Pennsylvania State University)