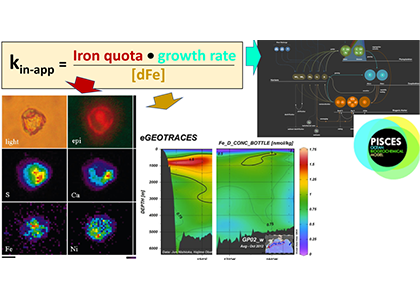
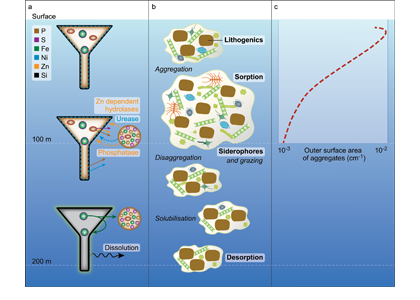
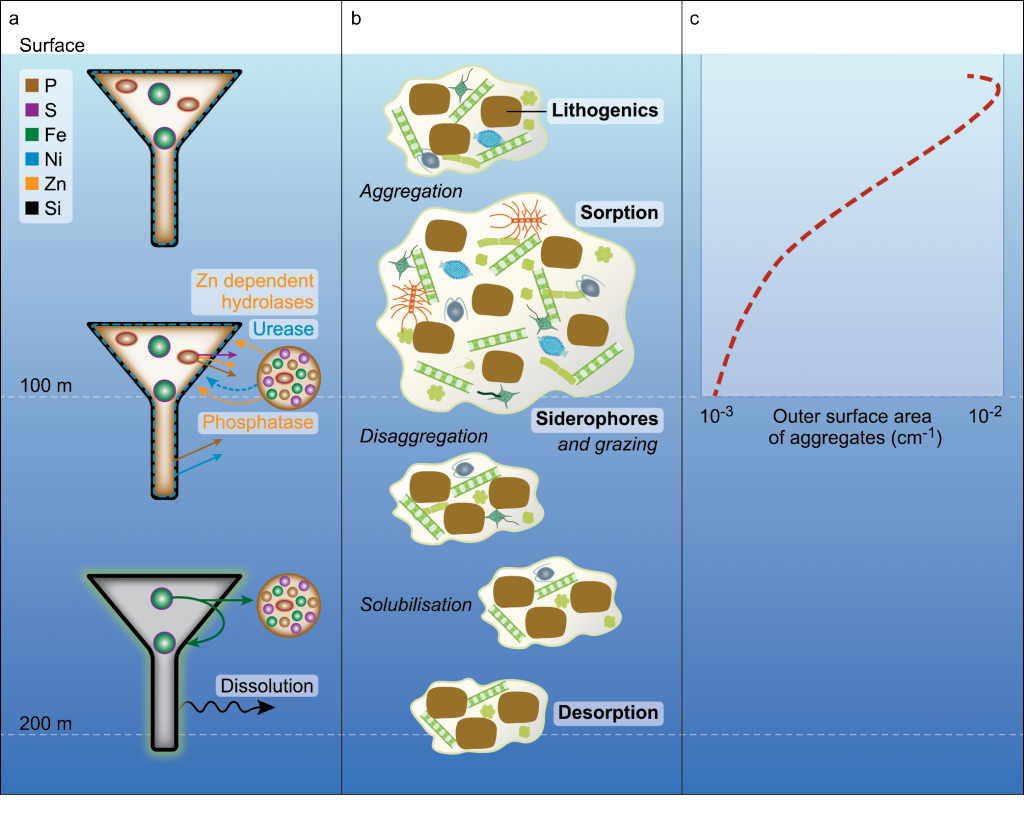
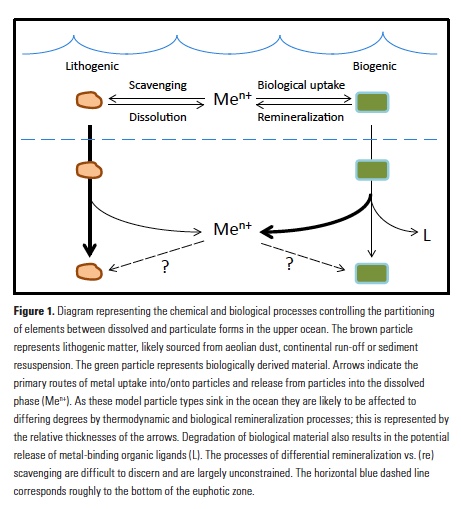
In many oceanic regions, iron exerts strong control on phytoplankton growth, ecosystem structure and carbon cycling. Yet, iron bioavailability and uptake rates by phytoplankton in the ocean are poorly constrained.
Recently, Shaked et al. (2020) (see GEOTRACES highlight), established a new approach for quantifying the availability of dissolved Fe (dFe) in natural seawater based on its uptake kinetics by Fe-limited cultured phytoplankton. In a follow up study published in GBC, this approach was extended to in situ phytoplankton, establishing a standardized proxy for dFe bioavailability in low-Fe ocean regions.
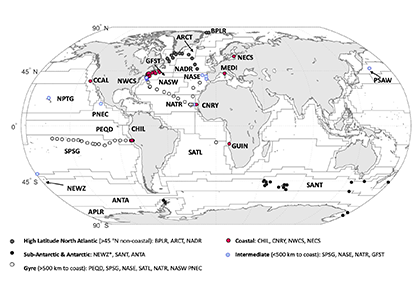
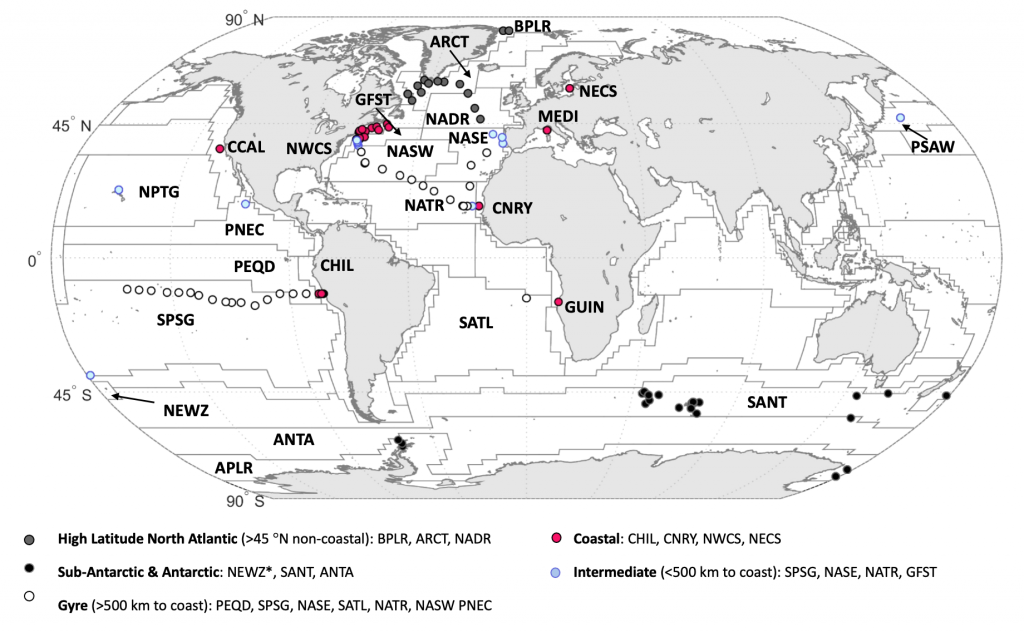
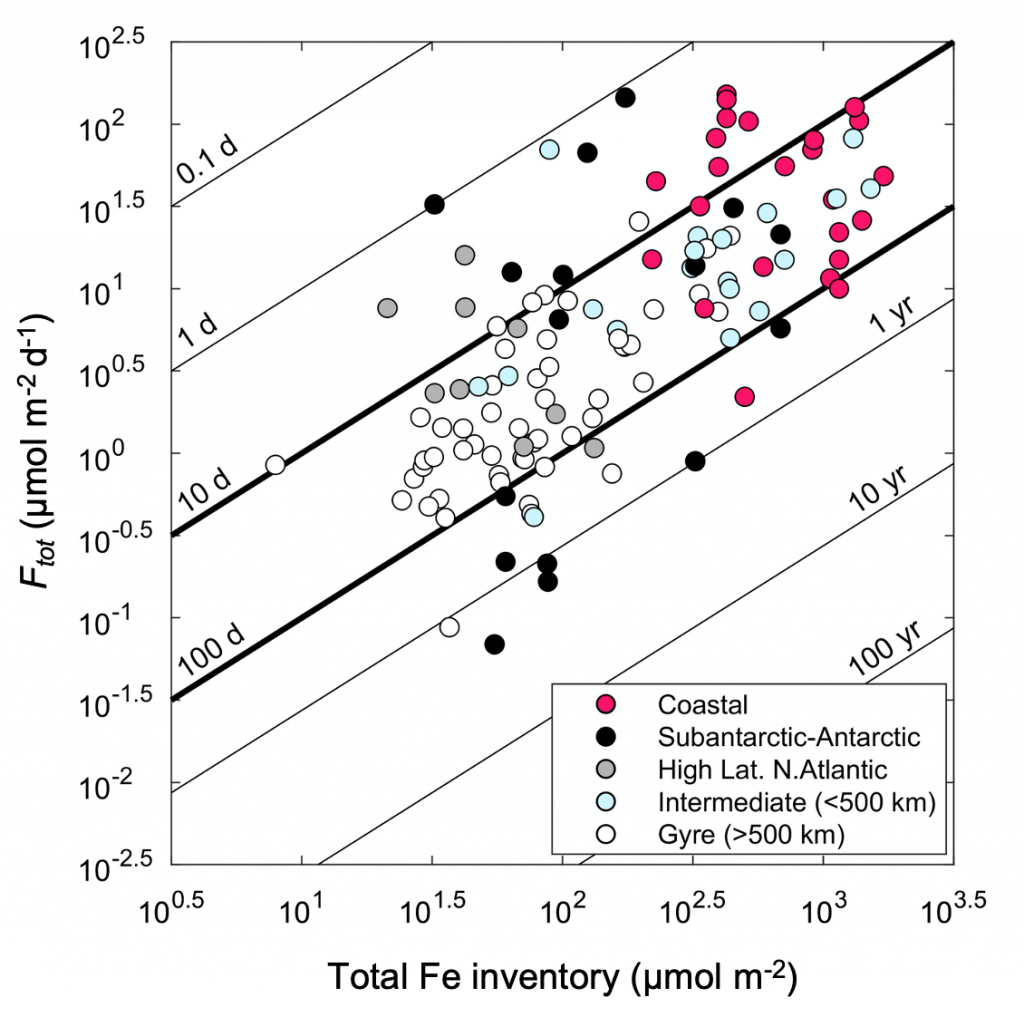
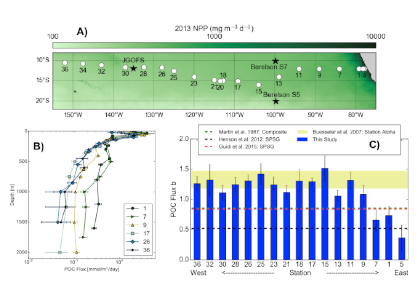
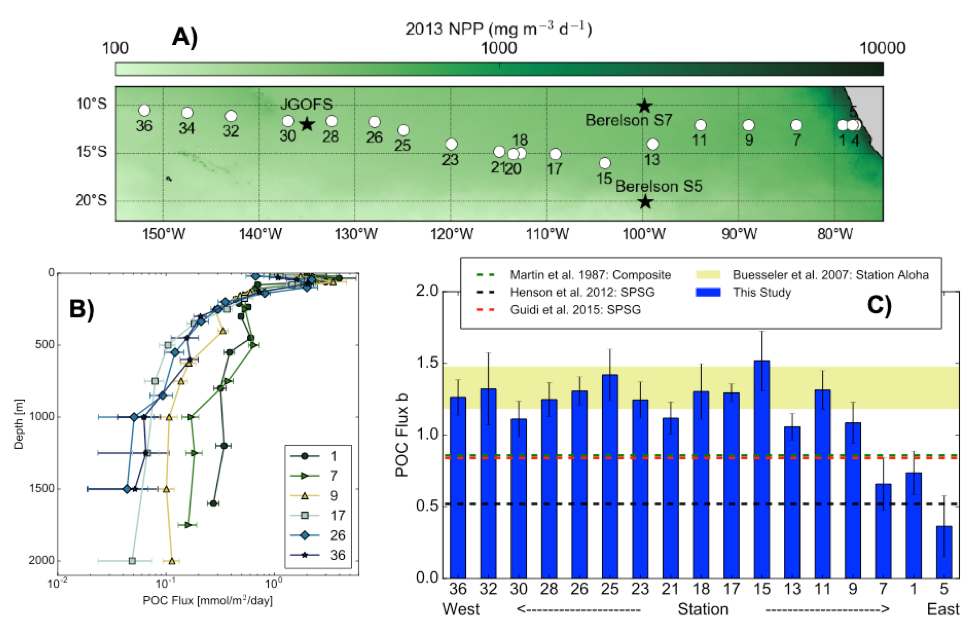
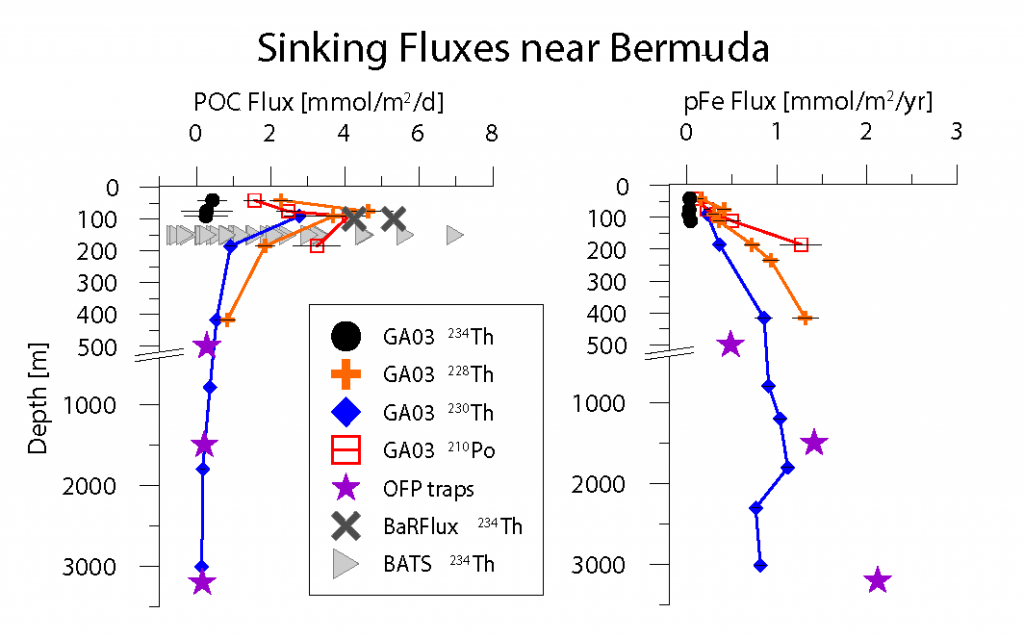
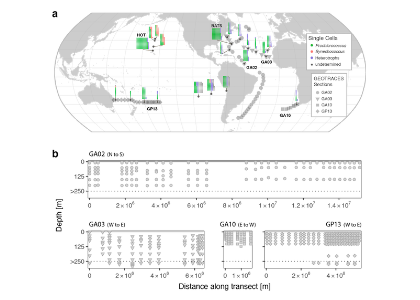
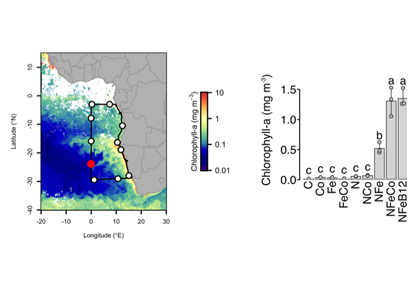
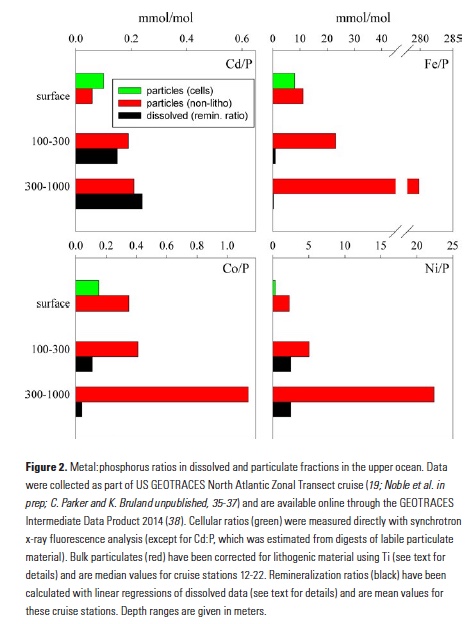
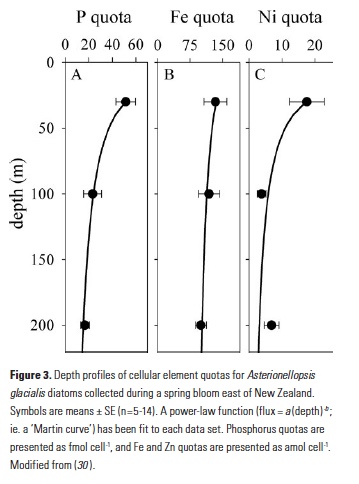
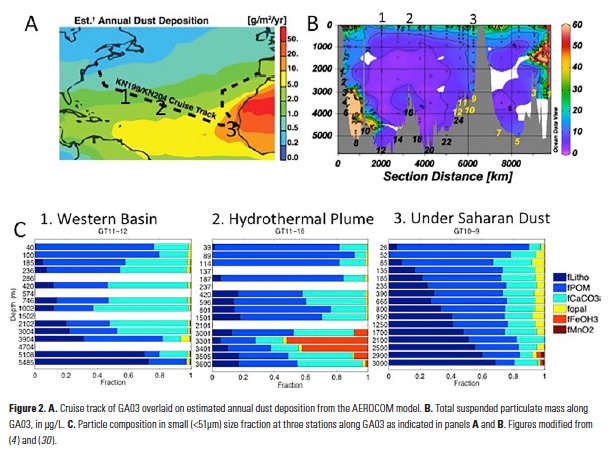
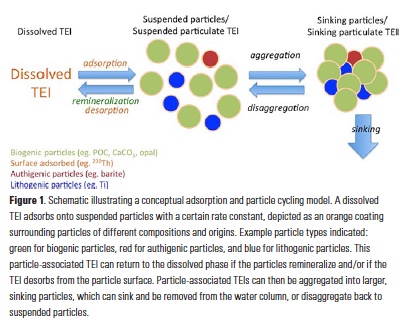
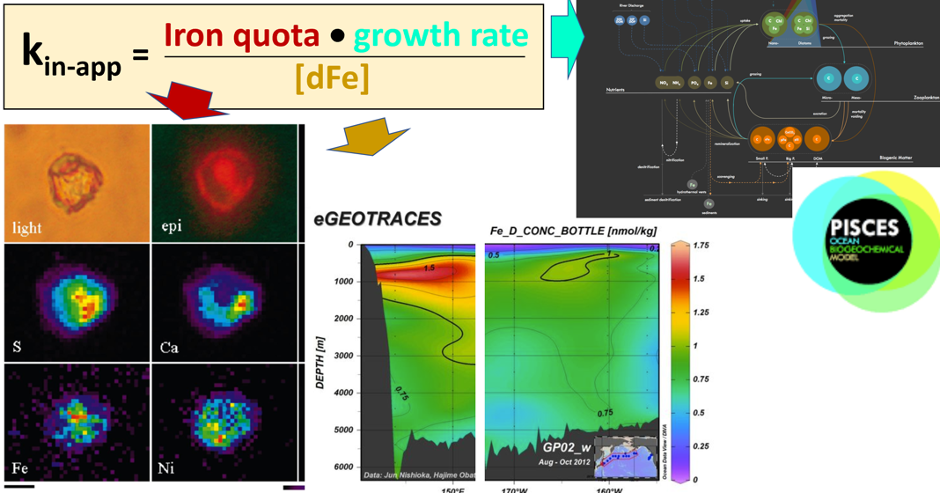
As explained in the short video lecture above, Yeala Shaked, Ben Twining, and their colleagues have analyzed large datasets collected during 10 research cruises (including 3 GEOTRACES section and process cruises) in multiple ocean regions. Dissolved Fe bioavailability was estimated through single cell Fe uptake rates, calculated by combining measured Fe contents of individual phytoplankton cells collected with concurrently-measured dFe concentrations, as well as modeled growth rates (Figure). Then the authors applied this proxy for: a) comparing dFe bioavailability among organisms and regions; b) calculating dFe uptake rates and residence times in low-Fe oceanic regions; and c) constraining Fe uptake parameters of earth system models to better predict ocean productivity in response to climate-change.
The data suggest that dFe species are highly available in low-Fe settings, likely due to photochemical reactions in sunlit waters.
Authors:
Y. Shaked (Hebrew University and Interuniversity Institute for Marine Sciences)
B.S. Twining (Bigelow Lab)
A. Tagliabue (University of Liverpool)
M.T. Maldonado (University of British Columbia)
K.N. Buck (University of South Florida)
T. Mellett (University of South Florida)
References:
Shaked, Y., Twining, B. S., Tagliabue, A., & Maldonado, M. T. (2021). Probing the bioavailability of dissolved iron to marine eukaryotic phytoplankton using in situ single cell iron quotas. Global Biogeochemical Cycles, e2021GB006979. https://doi.org/10.1029/2021GB006979
Shaked, Y., Buck, K. N., Mellett, T., & Maldonado, M. T. (2020). Insights into the bioavailability of oceanic dissolved Fe from phytoplankton uptake kinetics. The ISME Journal, 1–12. https://doi.org/10.1038/s41396-020-0597-3
Joint highlight with GEOTRACES – read here.