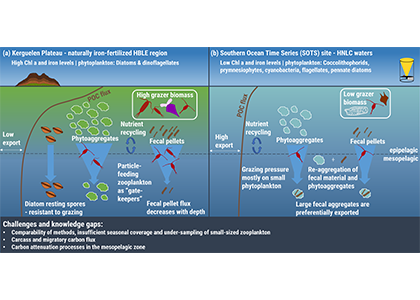
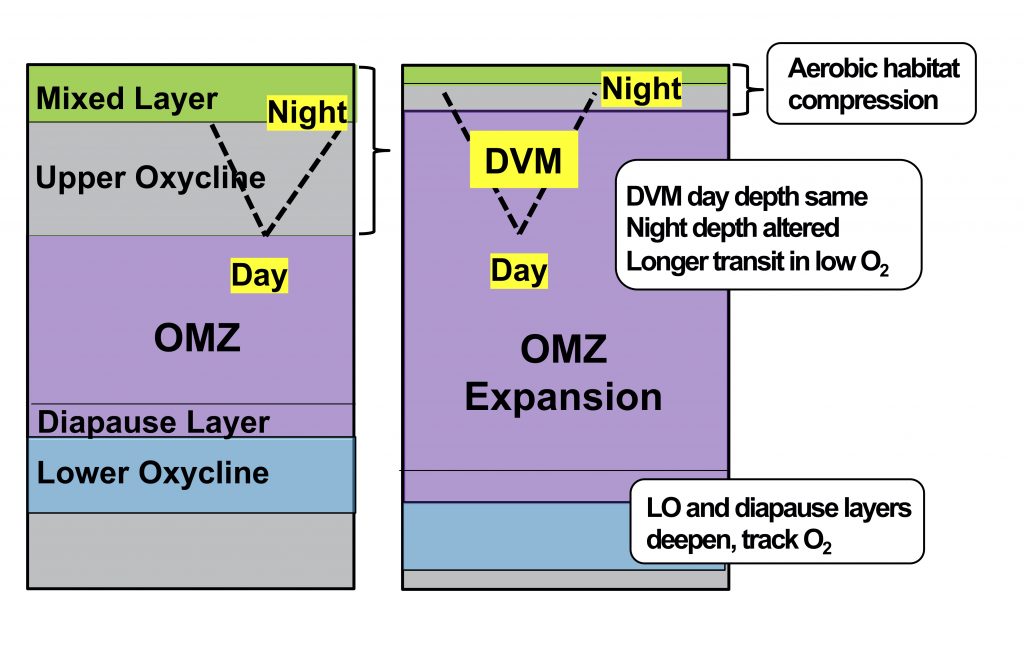
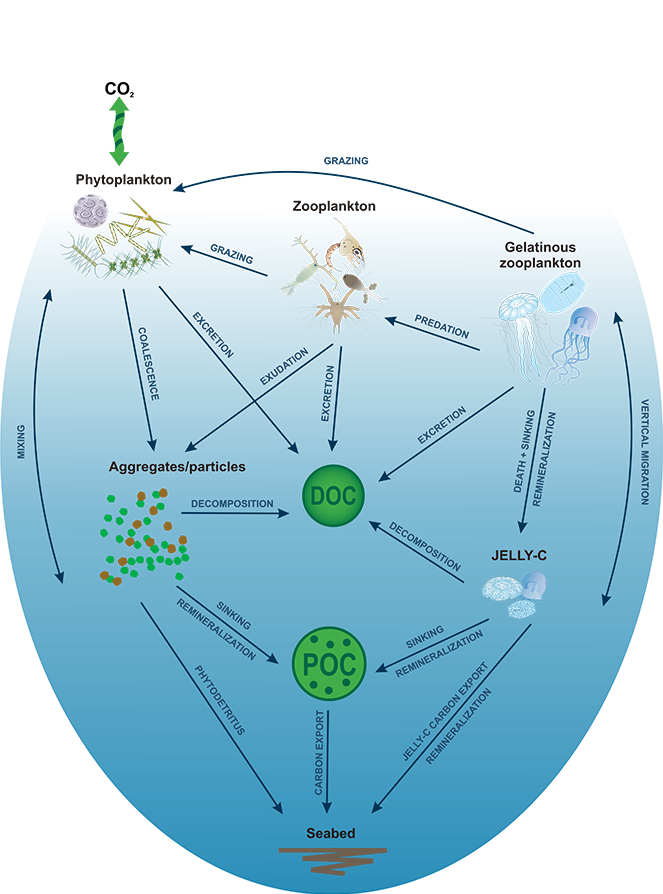
The Southern Ocean exhibits an inverse relationship between surface primary production and export flux out of the euphotic zone. The causes of this production-export decoupling are still under debate. A recently published mini review in Frontiers in Marine Science focused on zooplankton, an important component of Southern Ocean food webs and the biological pump. The authors compared carbon export regimes from the naturally iron-fertilised Kerguelen Plateau (high surface production, but generally low export) with the iron-limited and less productive high nutrient, low chlorophyll (HNLC) waters south of Australia, where carbon export is relatively high.
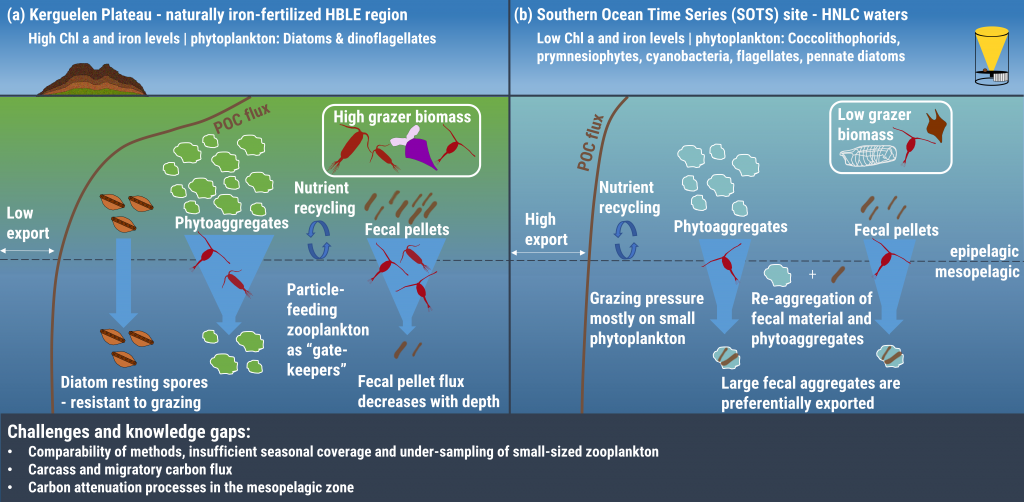
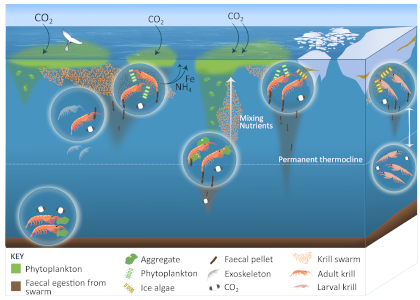
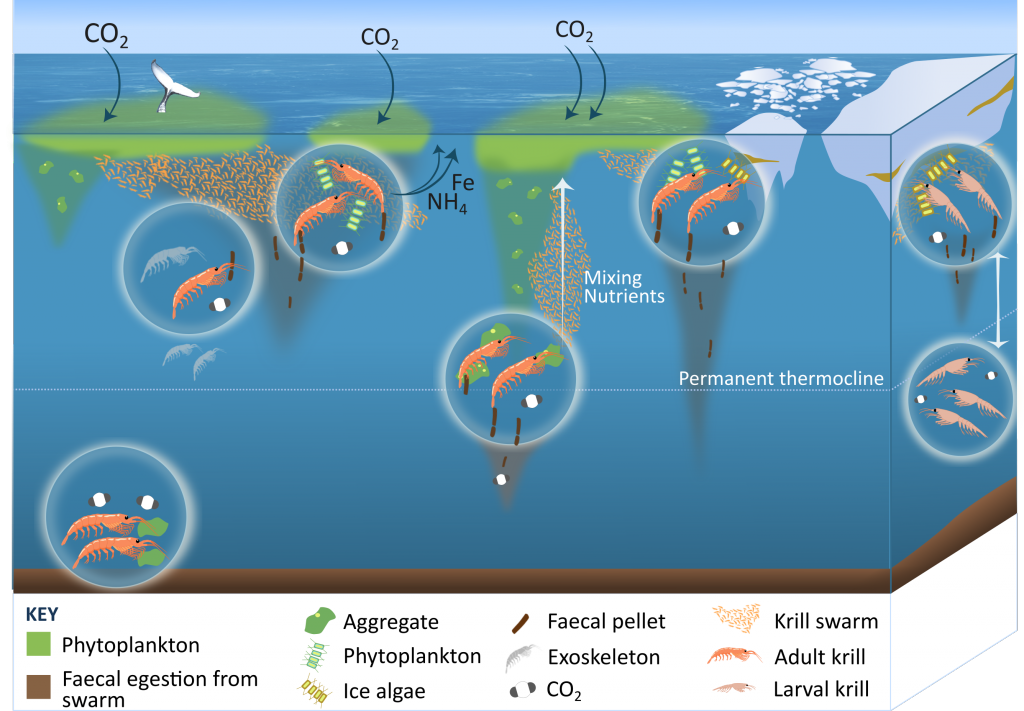
Figure 1: The role of zooplankton in establishing the characteristic export regimes at two sites in the Southern Ocean, (a) the highly productive northern Kerguelen Plateau, which exhibits low export, and (b) the iron-limited waters south of Australia with low production, but relatively high carbon export.
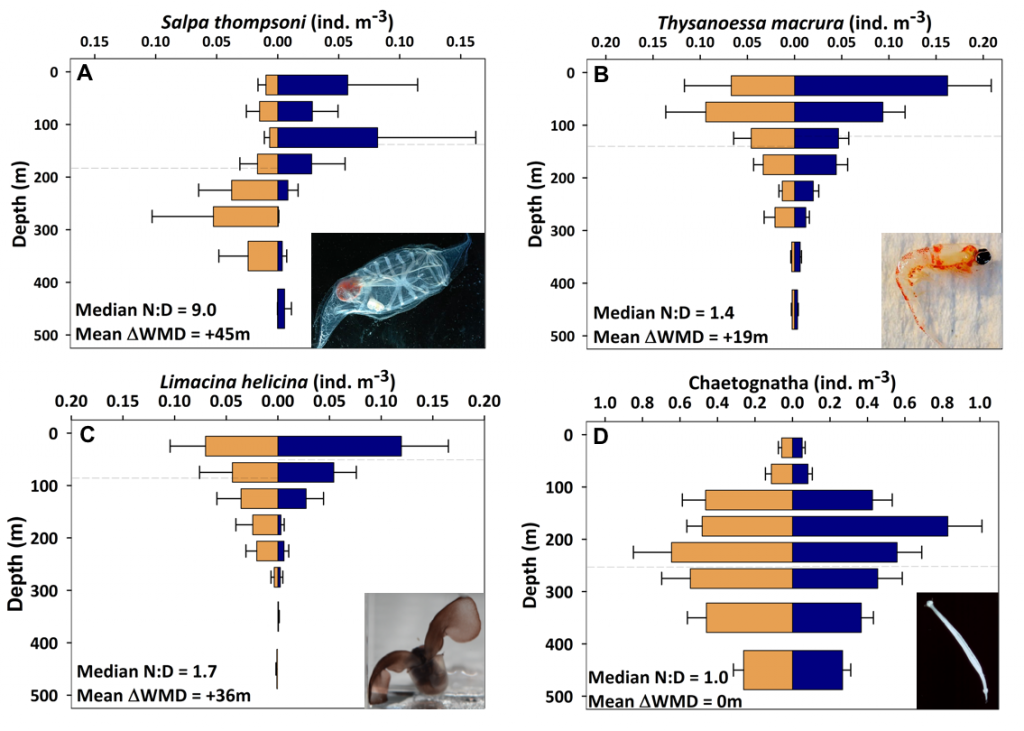
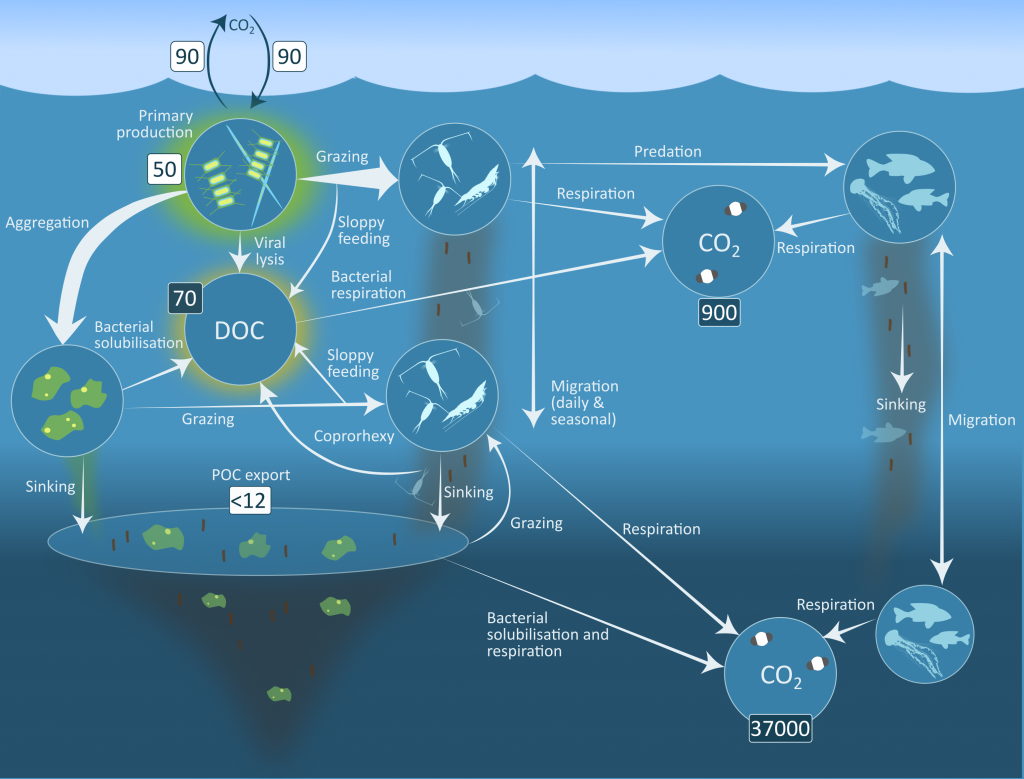
Size structure and zooplankton grazing pressure are found to shape carbon export at both sites. On the Kerguelen Plateau, a large size spectrum of zooplankton acts as “gate-keeper” to the mesopelagic by significantly reducing the sinking flux of phytoaggregates, which establishes the characteristic low export regime. In the HNLC waters, however, the zooplankton community is low in biomass and grazes predominantly on smaller particles, which leaves the larger particles for export and leads to relatively high export flux.
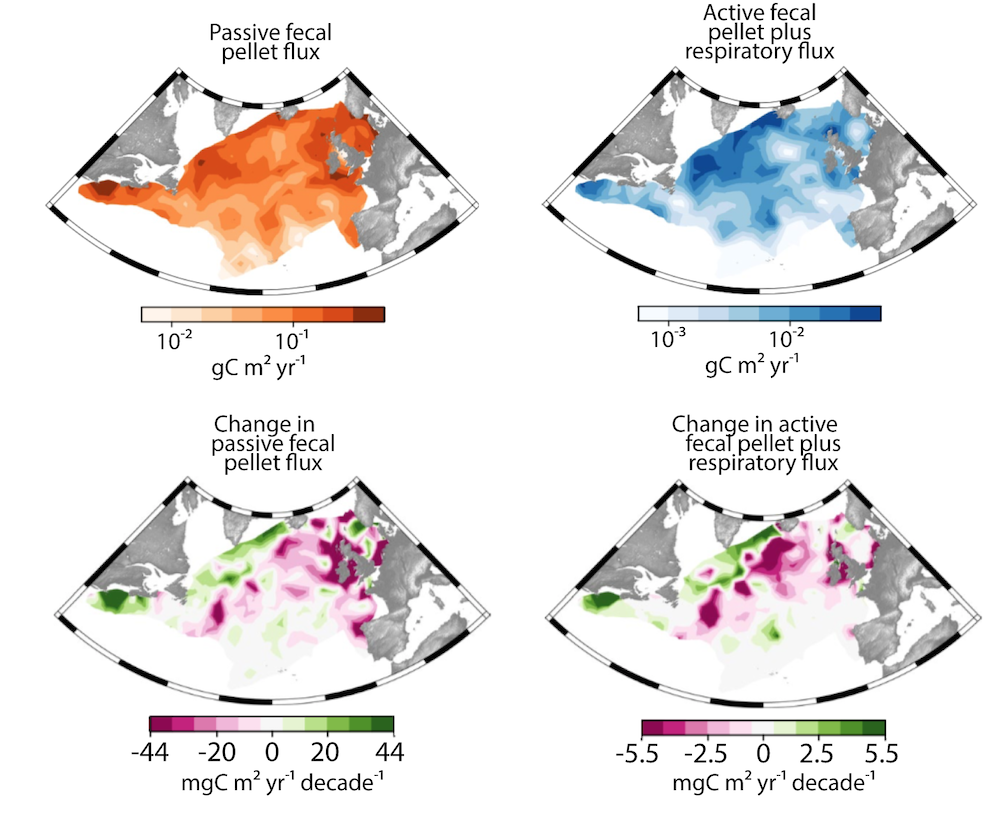
Gaps in knowledge related to insufficient seasonal data coverage, understudied carbon flux pathways, and associated mesopelagic processes limit our current understanding of carbon transfer through the water column and export. More integrated data collection efforts, including the use of autonomous profiling floats (e.g., BGC-Argo), stationary moorings, etc., will improve seasonal carbon flux data coverage, thus enabling more reliable estimation of carbon export and storage in the Southern Ocean and improved projection of future changes in carbon uptake and atmospheric carbon dioxide levels.
Authors:
Svenja Halfter (University of Tasmania)
Emma Cavan (Imperial College London)
Ruth Eriksen (CSIRO)
Kerrie Swadling (University of Tasmania)
Philip Boyd (University of Tasmania)