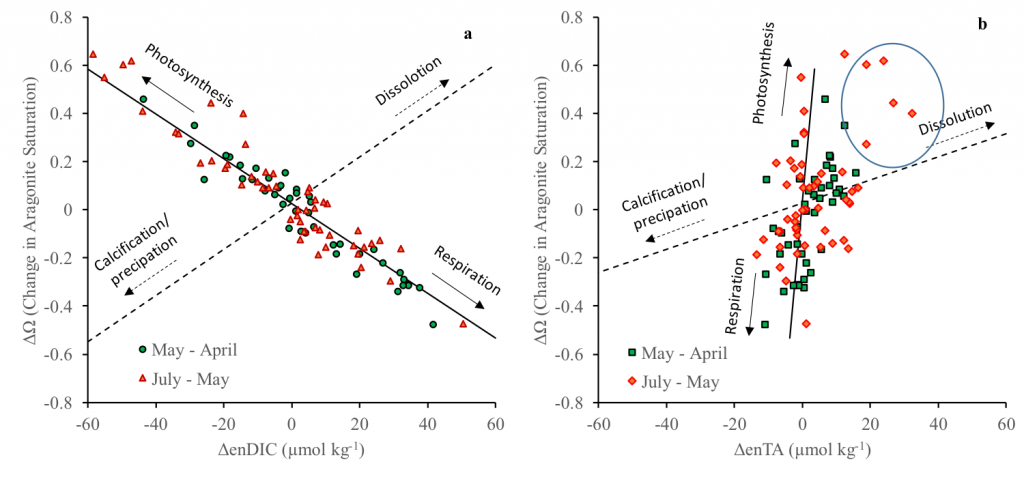
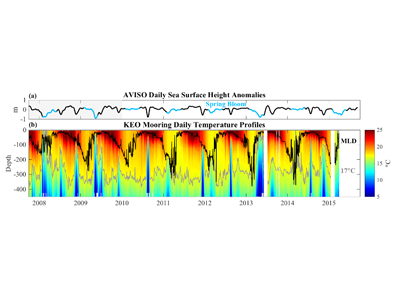
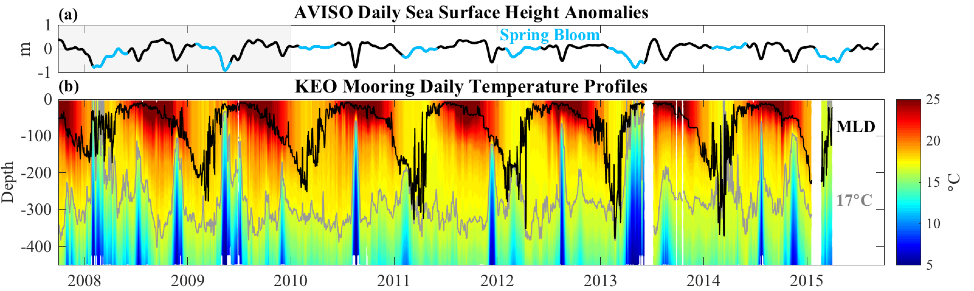
Coral reefs are diverse, productive ecosystems that are highly vulnerable to changing ocean conditions such as acidification and warming. Coral reef metabolism—in particular the fundamental ecosystem properties of net community production (NCP; the balance of photosynthesis and respiration) and net community calcification (NCC; the balance of calcification and dissolution)—has been proposed as a proxy for reef health. NCC is of particular interest, since ocean acidification is expected to have detrimental effects on reef calcification.
Traditionally, these metabolic rates are quantified through laborious methods that involve discrete sampling, which, due to a limited number of observations, often fails to characterize natural variability on time scales of minutes to days. In a recent paper in JGR, Takeshita et al. (2016) presented the Benthic Ecosystem and Acidification Measurement System (BEAMS), a fully autonomous system that simultaneously measures NCP and NCC at 15-minute intervals over a period of weeks. BEAMS utilizes the gradient flux method to quantify benthic metabolic rates by measuring chemical (pH and O2) and velocity gradients in the turbulent benthic boundary layer.

Two BEAMS were simultaneously deployed on Palmyra Atoll located approximately one km apart over vastly different benthic communities. One site was a healthy reef with approximately 70% coral cover, and the other was a degraded reef site with only 5% coral cover that was dominated by a non-calcifying invasive corallimorph Rhodactis howesii. Over the course of two weeks, BEAMS collected over 1,000 measurements of NCP and NCC from each site, yielding significantly different ratios of NCP to NCC between the two sites. These initial results suggest that BEAMS is capable of detecting different metabolic states, as well as patterns consistent with degrading reef health.
BEAMS is an exciting new autonomous tool to monitor reef health and study drivers of reef metabolism on timescales ranging from minutes to months (and potentially years). Additionally, autonomous measurement tools increase the potential for widespread and comparable observations across reefs and reef systems. Such knowledge will greatly improve our ability to predict the fate of coral reefs in a changing ocean.
Authors:
Yui Takeshita (Monterey Bay Aquarium Research Institute)


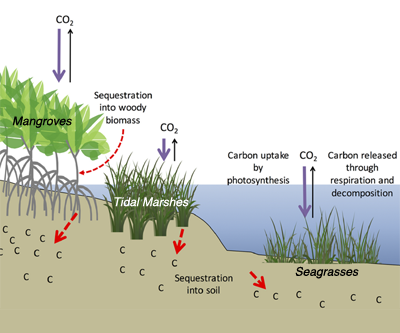
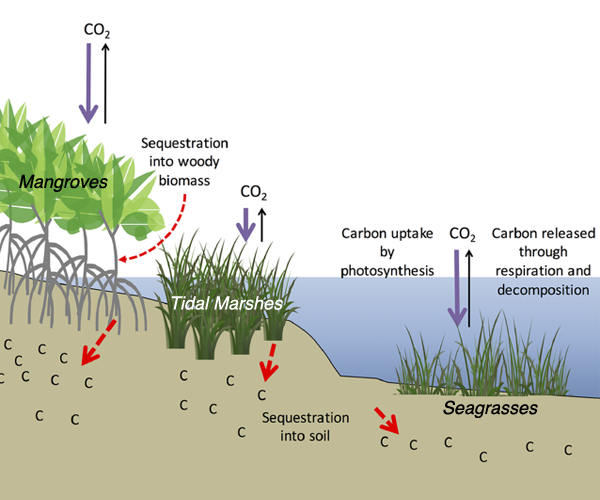

 Christiana Ade is a first-year PhD student at North Carolina State University in the Marine, Earth and Atmospheric Sciences Department. She researches wetlands and coastal environments using satellite remote sensing and field measurements. Her research includes water quality mapping, establishing new environmental indicators, and determining satellite resolution requirements for adequately monitoring wetlands.
Christiana Ade is a first-year PhD student at North Carolina State University in the Marine, Earth and Atmospheric Sciences Department. She researches wetlands and coastal environments using satellite remote sensing and field measurements. Her research includes water quality mapping, establishing new environmental indicators, and determining satellite resolution requirements for adequately monitoring wetlands. Henry Houskeeper
Henry Houskeeper  Suhey Ortiz Rosa is a PhD student conducting research with Dr. Roy Armstrong in Bio-Optical Oceanography at the Department of Marine Sciences at the University of Puerto Rico- Mayagüez (UPRM). In 2005, she completed a B.S. in Coastal Marine Biology at the University of Puerto Rico- Humacao, and in 2010, a MS in Chemical Oceanography at UPRM. Suhey’s work focuses on the biogeochemistry of coastal waters and coral reefs, validating algorithms from satellite imagery of complex optical waters, remote sensing, and GIS. Previously, she worked on CDOM characterization with PARAFAC, mapping marine species distribution with the GAP-Analysis Project of Puerto Rico and later with watershed analysis of sedimentation processes on coral reefs.
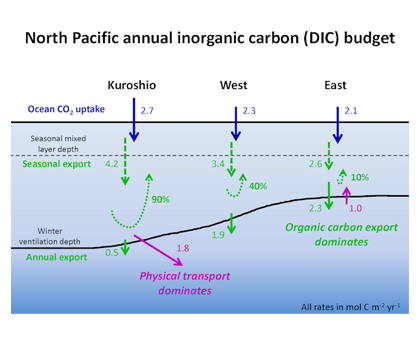
Suhey Ortiz Rosa is a PhD student conducting research with Dr. Roy Armstrong in Bio-Optical Oceanography at the Department of Marine Sciences at the University of Puerto Rico- Mayagüez (UPRM). In 2005, she completed a B.S. in Coastal Marine Biology at the University of Puerto Rico- Humacao, and in 2010, a MS in Chemical Oceanography at UPRM. Suhey’s work focuses on the biogeochemistry of coastal waters and coral reefs, validating algorithms from satellite imagery of complex optical waters, remote sensing, and GIS. Previously, she worked on CDOM characterization with PARAFAC, mapping marine species distribution with the GAP-Analysis Project of Puerto Rico and later with watershed analysis of sedimentation processes on coral reefs. Sara Rivero-Calle is a postdoctoral researcher at the Levine Lab in the University of Southern California interested in projects that involve large datasets, combining remote sensing and in situ data to answer large-scale ecological questions. She first learned about satellite remote sensing during her MS program at the University of Puerto Rico working on mesophotic reef sponge ecology using Autonomous Underwater Vehicles. She earned a PhD from Johns Hopkins University, where she used the Continuous Plankton Recorder survey to study long-term changes in North Atlantic phytoplankton communities. Currently, Sara is conducting postdoctoral research on fine-scale variability and patchiness, combining remote sensing, float, and HPLC data with numerical models.
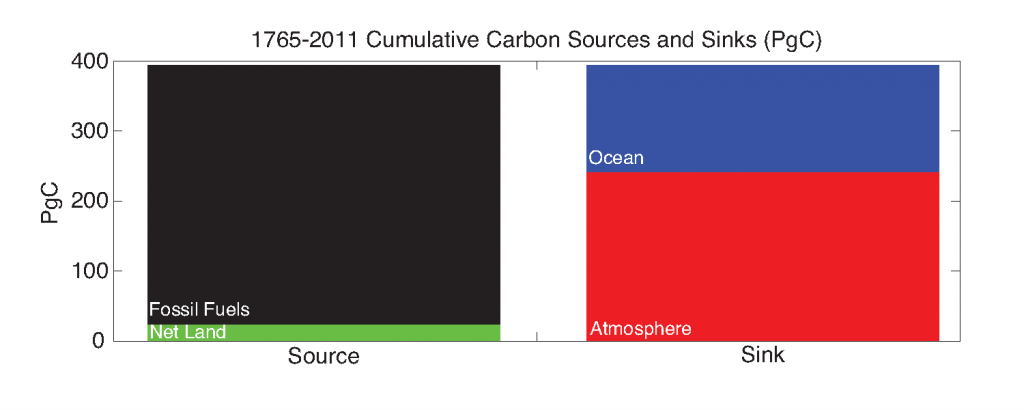
Sara Rivero-Calle is a postdoctoral researcher at the Levine Lab in the University of Southern California interested in projects that involve large datasets, combining remote sensing and in situ data to answer large-scale ecological questions. She first learned about satellite remote sensing during her MS program at the University of Puerto Rico working on mesophotic reef sponge ecology using Autonomous Underwater Vehicles. She earned a PhD from Johns Hopkins University, where she used the Continuous Plankton Recorder survey to study long-term changes in North Atlantic phytoplankton communities. Currently, Sara is conducting postdoctoral research on fine-scale variability and patchiness, combining remote sensing, float, and HPLC data with numerical models. Sarah Schlunegger is a PhD Student in the Program of Atmospheric and Oceanic Sciences, advised by Prof. Jorge Sarmiento. Sarah uses Earth System Models to predict the timing, sequence and inter-dependence of emerging anthropogenic signals in the ocean, with a focus on the ocean’s acquisition of anthropogenic carbon and heat. The ocean provides a climate service by absorbing the atmosphere’s excess carbon and heat but at a cost, namely acidification and warming, which deteriorate marine habitats. Sarah’s primary research goal is to identify when and where changes in these heat/carbon sinks and their resulting impacts will be detectable in the ocean.
Sarah Schlunegger is a PhD Student in the Program of Atmospheric and Oceanic Sciences, advised by Prof. Jorge Sarmiento. Sarah uses Earth System Models to predict the timing, sequence and inter-dependence of emerging anthropogenic signals in the ocean, with a focus on the ocean’s acquisition of anthropogenic carbon and heat. The ocean provides a climate service by absorbing the atmosphere’s excess carbon and heat but at a cost, namely acidification and warming, which deteriorate marine habitats. Sarah’s primary research goal is to identify when and where changes in these heat/carbon sinks and their resulting impacts will be detectable in the ocean.