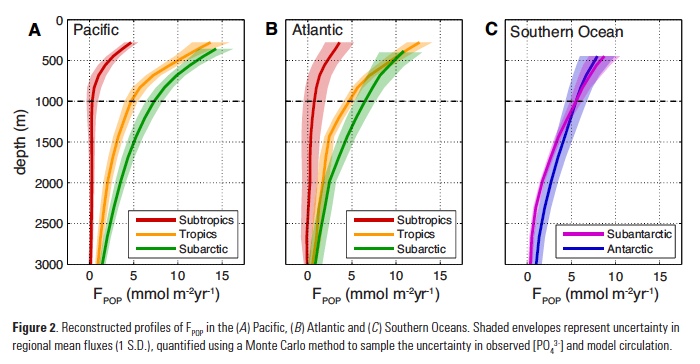
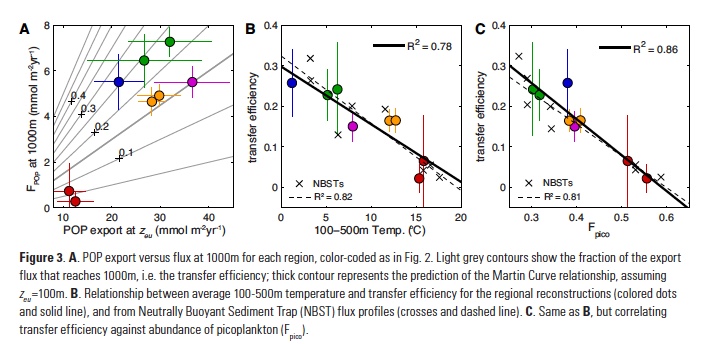
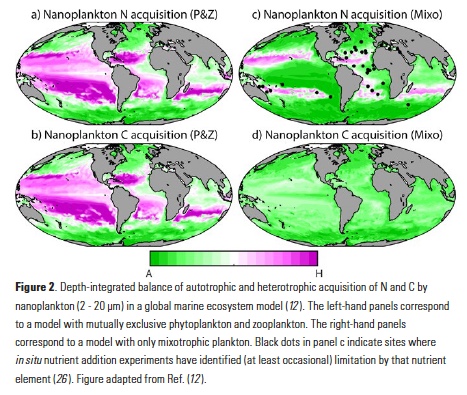
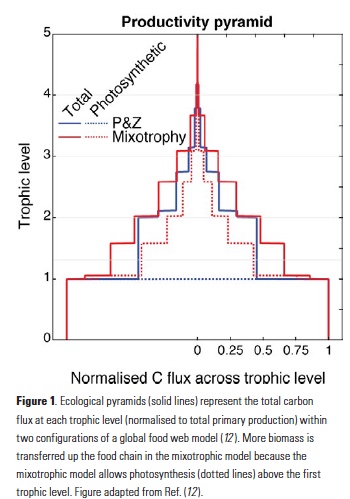
WBC Series Guest Editors: Andrea J. Fassbender1 and Stuart P. Bishop2
1. Monterey Bay Aquarium Research Institute
2. North Carolina State University
Western boundary current (WBC) regions are often studied for their intensity of air-sea interaction and mesoscale variability, yet research addressing the implications of these characteristics for biogeochemical cycling has lagged behind. WBCs, and their extension jets, display a wide breadth of physical processes that give rise to variability ranging from submesoscale (1-10 km) to basin scale (1000 km). WBC extension jets can act as both barriers and conduits for biological and chemical exchanges between subpolar-subtropical water masses, likely serving an important role in local chemical fluxes and biological community composition. Additionally, WBC regions are known for their formation of subtropical mode waters, carrying their source water biogeochemical signatures into the ocean interior. Interactions between (sub)mesoscale processes, mode water formation, and cross frontal exchanges of chemicals and organisms remain an important and nascent area of research.
In addition to the physical dynamics, many questions remain regarding the role of WBC regions in the global carbon cycle. Recent work suggests that these domains exhibit physically mediated export of biogenic particles and are gateways for anthropogenic carbon injection into the ocean interior. Such recent discovery that WBC processes may be strongly linked to the biological carbon pump and anthropogenic carbon storage speaks to the challenges associated with observing these ocean realms. While much has been learned from pairing satellite remote sensing with in situ physical oceanographic observations, biogeochemical analyses have historically been limited by the lack of necessary observing tools. Thus, there remains a critical knowledge gap on the role of WBCs in the global carbon cycle and other biogeochemical cycles.
With OceanObs’19 approximately two years away, the recent Ocean Carbon Hot Spots workshop assessed community interests and perspectives, revealing that it is an opportune time to make use of novel autonomous observing platforms and biogeochemical sensors to unravel some of the mysteries surrounding the role of WBC extensions in marine biogeochemical cycling. The articles herein present some of the most pressing research questions and observing hurdles related to WBCs from the perspectives of physical, chemical, and biological oceanographers and modelers working in this arena.
Series Articles:
Fine-scale biophysical controls on nutrient supply, phytoplankton community structure, and carbon export in western boundary current regions, S. Clayton, P. Gaube, T. Nagai, M.M. Omand, M. Honda
Decadal variability of the Kuroshio Extension system and its impact on subtropical mode water formation B. Qiu, E. Oka, S.P. Bishop, S. Chen, A.J. Fassbender
Western boundary currents as conduits for the ejection of anthropogenic carbon from the thermocline K.B. Rodgers, P. Zhai, D. Iudicone, O. Aumont, B. Carter, A. J. Fassbender, S. M. Griffies, Y. Plancherel, L. Resplandy, R.D. Slater, K. Toyama
The role of western boundary current regions in the global carbon cycle A.R. Gray, J. Palter
Observing air-sea interaction in the western boundary currents and their extension regions: Considerations for OceanObs 2019 D. Zhang, M.F. Cronin, X. Lin, R. Inoue, A.J. Fassbender, S.P. Bishop, A. Sutton